Profiles of cancer stem cell subpopulations in cholangiocarcinomas - PubMed (original) (raw)
. 2015 Jun;185(6):1724-39.
doi: 10.1016/j.ajpath.2015.02.010. Epub 2015 Apr 17.
Anastasia Renzi 2, Guido Carpino [ 3](#full-view-affiliation-3 "Movement, Human and Health Sciences, Division of Health Sciences, University of Rome "Foro Italico," Rome, Italy."), Alessia Torrice 1, Maria C Bragazzi 1, Felice Giuliante 4, Agostino M DeRose 4, Alice Fraveto 1, Paolo Onori 2, Chiara Napoletano 5, Antonio Franchitto 6, Alfredo Cantafora 1, GianLuca Grazi 7, Nicola Caporaso 8, Giuseppe D'Argenio 8, Gianfranco Alpini 9, Lola M Reid 10, Eugenio Gaudio 2, Domenico Alvaro 11
Affiliations
- PMID: 25892683
- PMCID: PMC4450332
- DOI: 10.1016/j.ajpath.2015.02.010
Profiles of cancer stem cell subpopulations in cholangiocarcinomas
Vincenzo Cardinale et al. Am J Pathol. 2015 Jun.
Abstract
Cholangiocarcinomas (CCAs) comprise a mucin-secreting form, intrahepatic or perihilar, and a mixed form located peripherally. We characterized cancer stem cells (CSCs) in CCA subtypes and evaluated their cancerogenic potential. CSC markers were investigated in 25 human CCAs in primary cultures and established cell lines. Tumorigenic potential was evaluated in vitro or in xenografted mice after s.c. or intrahepatic injection in normal and cirrhotic (carbon tetrachloride-induced) mice. CSCs comprised more than 30% of the tumor mass. Although the CSC profile was similar between mucin-intrahepatic and mucin-perihilar subtypes, CD13(+) CSCs characterized mixed-intrahepatic, whereas LGR5(+) characterized mucin-CCA subtypes. Many neoplastic cells expressed epithelial-mesenchymal transition markers and coexpressed mesenchymal and epithelial markers. In primary cultures, epithelial-mesenchymal transition markers, mesenchymal markers (vimentin, CD90), and CD13 largely predominated over epithelial markers (CD133, EpCAM, and LGR5). In vitro, CSCs expressing epithelial markers formed a higher number of spheroids than CD13(+) or CD90(+) CSCs. In s.c. tumor xenografts, tumors dominated by stromal markers were formed primarily by CD90(+) and CD13(+) cells. By contrast, in intrahepatic xenografts in cirrhotic livers, tumors were dominated by epithelial traits reproducing the original human CCAs. In conclusion, CSCs were rich in human CCAs, implicating CCAs as stem cell-based diseases. CSC subpopulations generate different types of cancers depending on the microenvironment. Remarkably, CSCs reproduce the original human CCAs when injected into cirrhotic livers.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
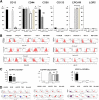
Flow cytometry (FC) analyses of established cell lines of human cholangiocarcinomas (CCAs). A: Bar graphs showing the distribution, as percentages, of cancer stem cell (CSC) subpopulation in established human cancer cell lines as assessed by FC for CSC markers. B: FC representative plots. We investigated: i) HuH-28 and CCLP-I (mucin-negative) and HUCCT-1 (mucin-positive) cell lines derived from human IHCCA; ii) TFK-1 (mucin-positive) cell line derived from pCCA; and iii) Mz-ChA-1 (mucin-positive) cell line derived from gallbladder carcinoma. CD44 expression is high in most of the cell lines (>74%). CD90+ cells are evident primarily in HuH-28 and CCLP-1. EpCAM positivity characterizes the predominant cell population in the intrahepatic (IHCCA) cell line, HUCCT-1, in perihilar (pCCA) cell lines, TFK-1 and, in gallbladder cancer cell line, Mz-ChA-1. CD133 and LGR5 are minimally expressed. C: Bar graphs showing the distribution, as percentage, of CSC subpopulations coexpressing CSC markers. D: FC representative plots. CD44+/CD90+ cells predominate in HuH-28 and CCLP-1 cell lines. CD44+/EpCAM+ cells are prevalent in HUCCT-1, TFK-1, and Mz-ChA-1 cell lines. CD13+/CD90+ cells predominate only in HuH-28 cells. ∗∗P < 0.01 versus the other cell lines; ††P < 0.01 versus HuH28.
Figure 2
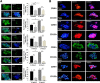
Immunophenotypic traits of human mucin-intrahepatic (IHCCA) and mixed-IHCCA. A: Periodic-acid Schiff (PAS) stain and immunohistochemistry (IHC) for keratin (K)7, EpCAM, LGR5, CD133, CD13, CD90, and α-smooth muscle actin (α-SMA) on human mucin-IHCCAs. Mucin-IHCCAs are diffusely positive for PAS, K7, EpCAM, LGR5, and CD133. By contrast, CD13 is restricted to a small subpopulation of tumor epithelial cells (arrow). CD90 and α-SMA are primarily expressed by tumor stromal cells (arrows). B: PAS stain and IHC for K7, EpCAM, LGR5, CD133, CD13, CD90, and α-SMA on human mixed-IHCCAs. Mixed-IHCCAs are diffusely positive for K7, EpCAM, CD13, and CD133. By contrast, LGR5 is restricted to a small subpopulation of tumor epithelial cells (arrows). CD90 and α-SMA were primarily expressed by tumor stromal cells (arrows). C: Double immunofluorescence (IF) for Desmin (red) and CD133 (green) in mucin-IHCCA; double IF for α-SMA (red) and pan-cytokeratin (Pan-CK; green) in mixed-IHCCAs. Approximately 10% of tumor epithelial cells (CD133+ or Pan-CK+, in green) express mesenchymal markers (desmin or α-SMA, in red) both in mucin- and in mixed-IHCCA. Yellow arrows indicate cells coexpressing epithelial and mesenchymal markers (yellow cells in merged images). Nuclei are shown in blue. Separate channels are shown. Boxed areas are shown magnified in the insets (bottom panels). D: Upper panels: IHC for SNAIL and Twist in human CCA specimens shows the expression of the two antigens within the nuclei of epithelial CCA cells (arrows). Lower panels: Double IF for SNAIL (red) and Pan-CK (green) in human CCA specimens confirms the expression of SNAIL within nuclei of epithelial CCA cells (arrows). Double IF for SNAIL (red) and α-SMA (green) in human CCA specimens shows the presence of CCA cells expressing both α-SMA and SNAIL. Images from mixed-IHCCA samples are displayed. E–J: IHC for CD133, CD13, and CD90 on human nontumor tissue surrounding CCA specimens. CD133 expression (E and F) is restricted to a few cells within Canals of Hering (CoH) and bile ductules (E, arrow) and to peribiliary glands (PBGs) within larger bile ducts (F, arrows). CD90 (G and H) is mostly expressed by stromal cells in the portal spaces (G, arrow) and around PBGs (H, arrows). CD13 (I and J) is diffusely expressed by hepatocytes, cells within CoH/bile ductules (I, yellow arrows), cholangiocytes of interlobular bile ducts (I, red arrow), and PBGs (J, arrow). Original magnification: ×20 (A–C, D, lower panels); ×40 (D, upper panel).
Figure 3
Human cholangiocarcinoma (CCA) primary cultures. A: Immunohistochemical analyses of CCA primary cultures after 2 to 3 passages (P2/P3) and 20 to 30 passages (P > 20). The expression of mesenchymal and epithelial-mesenchymal transition markers (vimentin, α-SMA, SNAIL, Twist, S100A4, P-cadherin) largely predominate over that of epithelial markers (CD133, EpCAM, LGR5, E-cadherin) in all passages examined. During progression of primary cultures (P > 20 versus P2/P3) SNAIL- and Twist-positive cells tend to increase, whereas EpCAM-, LGR5-, and CD133-positive cells tend to decrease. Similar diffuse positivity for vimentin, α-SMA, and S100A4 is observed in all passages. B–D: Flow cytometric (FC) analyses of primary cultures (20 to 30 passages) obtained from mixed-IHCCA (IH-mixed), mucin-IHCCA (IH-mucin), and mucin-pCCA (p-mucin) subtypes; representative FC plots. B: Cells positive for CD13, CD44, and CD90 largely predominate with respect to CD133, EpCAM, and LGR5. CD13+ and CD44+ cells predominate in mixed-IHCCA with respect to mucin-IHCCA or mucin-pCCA, whereas the opposite is found for CD90+ cells. C: Cells double-positive for CD13 and CD44 (CD13+/CD44+) predominate in mixed-IHCCA with respect to mucin-IHCCA or mucin-pCCA. D: CD90+/CD44−/CD13− cells predominate in mucin-IHCCAs and mucin-pCCA with respect to mixed-IHCCAs. In these experiments, CD90+ cells are gated and further analyzed for the expression of CD44 and CD13 markers. ∗P < 0.05, ∗∗P < 0.01 versus mucin-CCAs. Original magnification: ×40 (A).
Figure 4
Spheroids formed by cholangiocarcinomas (CCAs) subpopulations. A: We determined the capacity of spheroid formation by cancer stem cell (CSC) subpopulations immunosorted from CCA primary cultures; the number of spheroids (see bar graph) formed in vitro being a self-renewal index. By immunofluorescence (IF) for the marker used for immunosorting, we tested the purity of the formed spheroids. Spheroids formed by CD90+ cells are enriched for CD90+ cells, whereas the negative counterpart CD90− forms spheroids where CD90+ cells are <5%, indicating that these spheroids are formed by other CSC subpopulations. Each subpopulation forms spheroids efficiently, reaching a size of 100 to 500 μm after 7 days in culture. IF (nuclei were stained with DAPI) shows the enrichment in the spheroids by the immunosorted cell subpopulation. CD133, EpCAM, and LGR5 form a higher (P_ < 0.01) number of spheroids compared to cells expressing the mesenchymal cell marker, CD90, or CD13 (note that scales are different). CD13+ cells from mixed-IHCCA (IH-mixed) form a higher number of spheroids than CD13+ cells immunoselected from mucin-IHCCA (IH-mucin), whereas the opposite is observed for CD90+ or CD133+ cells (mucin > mixed.) B: Spheroids formed by CD133+ and CD90+ CSC subpopulations were analyzed by IF for markers of epithelial-mesenchymal transition (Twist, SNAIL, vimentin, P-cadherin). Spheroids show positive staining for Twist, SNAIL, vimentin, and P-cadherin without differences between CD133+ and CD90+ spheroids. ∗_P < 0.05, ∗∗P < 0.01. Original magnification: ×30 (A); ×40 (B).
Figure 5
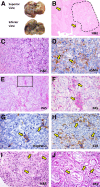
Histology of s.c. human tumor xenografts. A: Hematoxylin and eosin (H&E): Xenografts arising from CD90+, EpCAM+, and LGR5+ spheroids are characterized by the presence of larger necrotic areas in comparison with tumors obtained from CD133+ cells, whereas in xenografts from CD13+ spheroids, only few and restricted necrotic areas are observed. Necrotic areas are encircled by the dotted line. B: H&E: All s.c. xenografts comprise of nests of pleomorphic cells with giant and irregular nuclei and multiple prominent nucleoli. Numerous tumor cells are PCNA positive. Most tumor cells are α-SMA positive (arrows), whereas few and scattered nests of tumor cells express K19 (arrows). C: Spheroids from mucin-IHCCA, injected s.c., give rise to xenografts in which neoplastic cells are surrounded by a Periodic-acid Schiff (PAS)-positive material (arrows). D: EpCAM+ and LGR5+ spheroids from mucin-IHCCA show the presence of few areas composed of K19+ duct-like structures (arrows). E: Spheroids from mixed-IHCCA give rise to xenografts that are almost PAS negative. F: 5% to 30% of cells are K19 positive and form few ductular-like structures (arrows). Original magnification: ×10 (A); ×40 (B–F).
Figure 6
Intrahepatic tumor xenografts: morphological and phenotypic features. Injection of spheroids (approximately 10,000 cells) formed by cells immunoselected for a determined cholangiocarcinoma (CCA) marker into the livers of cirrhotic SCID mice (carbon tetrachloride–induced) leads, after 4 weeks, to evident liver cancers. A: Tumor masses observed 4 weeks after intrahepatic injection of CD90+ spheroids from primary cultures of mucin-IHCCA. B: Hematoxylin and eosin (H&E): The liver is occupied by several tumor masses (dotted line); vascular invasion (arrows). C: H&E: Tumor masses were composed of strands of polygonal cells with giant nuclei and prominent nucleoli. D: Immunohistochemistry (IHC): At the center of tumor masses, α-SMA–positive tumor cells are present (arrows). E–H: At the periphery of the tumor masses, Periodic-acid Schiff (PAS)-positive cells (F, arrows), cords of HepPar-1–positive cells (G, arrows), and K19 ductular-like structures (H, arrows) are present, reproducing a moderately differentiated carcinoma. E = PAS staining: Area in the box is magnified in F; F = PAS staining: G = IHC for HepPar-1. H = IHC for CK19. I and J: Immunocompetent cirrhotic BALBc mice, under pharmacological immunosuppression, injected with CD133+ spheroids prepared from mucin-IHCCA primary cultures. Four weeks after intrahepatic injection, a tumor almost totally composed of PAS+ duct-like structures (arrows) is observed. I = H&E: J = PAS staining. n = 4 (I and J, mucin-IHCCA primary cultures). Original magnification: ×10 (B and E); ×20 (C, D, H, and I); ×40 (F, G, and J).
Similar articles
- Isolation and characterization of tumorigenic extrahepatic cholangiocarcinoma cells with stem cell-like properties.
Wang M, Xiao J, Shen M, Yahong Y, Tian R, Zhu F, Jiang J, Du Z, Hu J, Liu W, Qin R. Wang M, et al. Int J Cancer. 2011 Jan 1;128(1):72-81. doi: 10.1002/ijc.25317. Int J Cancer. 2011. PMID: 20232394 - Role of Cancer Stem Cells in Cholangiocarcinoma and Therapeutic Implications.
Wu HJ, Chu PY. Wu HJ, et al. Int J Mol Sci. 2019 Aug 25;20(17):4154. doi: 10.3390/ijms20174154. Int J Mol Sci. 2019. PMID: 31450710 Free PMC article. Review. - Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma.
Raggi C, Taddei ML, Sacco E, Navari N, Correnti M, Piombanti B, Pastore M, Campani C, Pranzini E, Iorio J, Lori G, Lottini T, Peano C, Cibella J, Lewinska M, Andersen JB, di Tommaso L, Viganò L, Di Maira G, Madiai S, Ramazzotti M, Orlandi I, Arcangeli A, Chiarugi P, Marra F. Raggi C, et al. J Hepatol. 2021 Jun;74(6):1373-1385. doi: 10.1016/j.jhep.2020.12.031. Epub 2021 Jan 21. J Hepatol. 2021. PMID: 33484774 - Lgr5+CD44+EpCAM+ Strictly Defines Cancer Stem Cells in Human Colorectal Cancer.
Leng Z, Xia Q, Chen J, Li Y, Xu J, Zhao E, Zheng H, Ai W, Dong J. Leng Z, et al. Cell Physiol Biochem. 2018;46(2):860-872. doi: 10.1159/000488743. Epub 2018 Mar 29. Cell Physiol Biochem. 2018. PMID: 29627827 - Tumor Microenvironment Versus Cancer Stem Cells in Cholangiocarcinoma: Synergistic Effects?
Romano M, De Francesco F, Gringeri E, Giordano A, Ferraro GA, Di Domenico M, Cillo U. Romano M, et al. J Cell Physiol. 2016 Apr;231(4):768-76. doi: 10.1002/jcp.25190. Epub 2015 Sep 22. J Cell Physiol. 2016. PMID: 26357947 Review.
Cited by
- Cell of origin in biliary tract cancers and clinical implications.
Moeini A, Haber PK, Sia D. Moeini A, et al. JHEP Rep. 2021 Jan 19;3(2):100226. doi: 10.1016/j.jhepr.2021.100226. eCollection 2021 Apr. JHEP Rep. 2021. PMID: 33665585 Free PMC article. Review. - Prognostic Role of Immune Checkpoint Regulators in Cholangiocarcinoma: A Pilot Study.
Cao L, Prithviraj P, Shrestha R, Sharma R, Anaka M, Bridle KR, Kannourakis G, Crawford DHG, Jayachandran A. Cao L, et al. J Clin Med. 2021 May 19;10(10):2191. doi: 10.3390/jcm10102191. J Clin Med. 2021. PMID: 34069452 Free PMC article. - Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA).
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Banales JM, et al. Nat Rev Gastroenterol Hepatol. 2016 May;13(5):261-80. doi: 10.1038/nrgastro.2016.51. Epub 2016 Apr 20. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27095655 - Exploring the role of tumor stemness and the potential of stemness-related risk model in the prognosis of intrahepatic cholangiocarcinoma.
Yue Y, Tao J, An D, Shi L. Yue Y, et al. Front Genet. 2023 Jan 12;13:1089405. doi: 10.3389/fgene.2022.1089405. eCollection 2022. Front Genet. 2023. PMID: 36712866 Free PMC article. - Advances of cancer-associated fibroblasts in liver cancer.
Peng H, Zhu E, Zhang Y. Peng H, et al. Biomark Res. 2022 Aug 16;10(1):59. doi: 10.1186/s40364-022-00406-z. Biomark Res. 2022. PMID: 35971182 Free PMC article. Review.
References
- Komuta M., Govaere O., Vandecaveye V., Akiba J., Van Steenbergen W., Verslype C., Laleman W., Pirenne J., Aerts R., Yano H., Nevens F., Topal B., Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA182322/CA/NCI NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- DK58411/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 DK058411/DK/NIDDK NIH HHS/United States
- R01 DK054811/DK/NIDDK NIH HHS/United States
- 1R21CA182322-01/CA/NCI NIH HHS/United States
- DK062975/DK/NIDDK NIH HHS/United States
- I01 BX000574/BX/BLRD VA/United States
- CA016086/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous