CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature - PubMed (original) (raw)
CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature
Sharon E Miller et al. Dev Cell. 2015.
Abstract
The size of endocytic clathrin-coated vesicles (CCVs) is remarkably uniform, suggesting that it is optimized to achieve the appropriate levels of cargo and lipid internalization. The three most abundant proteins in mammalian endocytic CCVs are clathrin and the two cargo-selecting, clathrin adaptors, CALM and AP2. Here we demonstrate that depletion of CALM causes a substantial increase in the ratio of "open" clathrin-coated pits (CCPs) to "necked"/"closed" CCVs and a doubling of CCP/CCV diameter, whereas AP2 depletion has opposite effects. Depletion of either adaptor, however, significantly inhibits endocytosis of transferrin and epidermal growth factor. The phenotypic effects of CALM depletion can be rescued by re-expression of wild-type CALM, but not with CALM that lacks a functional N-terminal, membrane-inserting, curvature-sensing/driving amphipathic helix, the existence and properties of which are demonstrated. CALM is thus a major factor in controlling CCV size and maturation and hence in determining the rates of endocytic cargo uptake.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
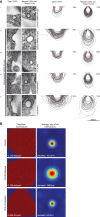
CALM Is Important for CCP and CCV Size Morphology (A) All cells express an HA-tagged version of the endocytic SNARE VAMP8. The size variation of clathrin-coated structures, as a consequence of CALM depletion without and with re-expression of WT and mutant versions of CALM (ii–iv), compared to those found in control cells (i) are shown and quantified. Left-hand columns show representative images of open and necked/closed clathrin-coated structures for each cell line. Right-hand columns show the traces of 100 randomly selected clathrin-coated structures and the percentage (∼150 images) of each type of structure adjacent to the traces. Dotted red line represents average size in control cells. Scale bars represent 200 nm. (B) Cells were stained with antibodies for clathrin, AP2, and CALM and appropriate fluorochrome-conjugated secondary antibodies. After acquisition of confocal images, STED images were collected for clathin and processed for quantification, as described in Experimental Procedures. Images on the left display all identified clathrin-stained CCPs/CCVs in a polygonal area defined by the outermost objects and the calculated number/μm2. Images on the right show a heat map of the overlay of all CCPs/CCVs identified (image on the left) and the calculated average diameter.
Figure 2
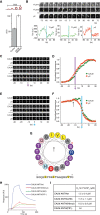
CALM Affects CCV Maturation and Possesses an Amphipathic Helix that Contributes to Membrane Binding (A) The time between first detection of a CCP and the first detected scission event at that CCP (Δts) was significantly extended in cells treated with CALM siRNA (control cells, Δt = 59 s, SEM = 3 s, 688 events, five cells; CALM siRNA, Δt = 100 s, SEM = 4 s, 590 events, five cells). (B) An example CCP and scission event from a control cell. Images were acquired using TIRF microscopy and the pulsed pH protocol to detect single scission events. Images acquired at pH 7 (upper, Tf pH7) show a cluster of Transferrin-phluorin (Tf-phl) at a CCP. A scission event was detected when Tf-phl at the cluster became insulated from the externally imposed pH change (middle, Tf pH5, t = 0 s). Simultaneous detection of μ2-mCherry showed this CCP formed ∼62 s before the detected scission event (lower, arrow in μ2-mCherry graph). The quantified fluorescence changes for this example CCP are plotted (graphs). (C) Montages of example clathrin-coated pit nucleation events imaged using TIRF microscopy. The purple arrow indicates t = 0 s, the first frame in which the CALM-GFP (green) object was detected using automated tracking. (D) Average CALM-GFP and μ2-mCherry (red) fluorescence traces of nucleating CCPs aligned to the first frame of detection of CALM-GFP (green). The average traces were normalized between the average of the first three fluorescence values and the peak fluorescence value for CALM and μ2, respectively. The fluorescence traces have a characteristic sigmoidal shape. (E) Montages of example clathrin-coated pit budding events. The blue arrow indicates t = 0 s, the moment of maximum decrease in CALM-GFP fluorescence that corresponds to vesicle scission. (F) Average CALM-GFP and μ2-mCherry at budding clathrin-coated pits. The average fluorescence traces were normalized to the average of the first three and last three fluorescence values for CALM and μ2, respectively. The μ2-mCherry fluorescence starts to decrease ∼15 s before the CALM-GFP signal (black arrow). (G) Heliquest server (
) helical wheel shows the orientation of the key hydrophobic residues (in yellow) and predicts the N terminus of CALM is an amphipathic helix with curvature-sensing properties. The hydrophobic moment points up toward the membrane and polar but mainly uncharged side chains are disposed laterally along where the surface the membrane would be. The negative charge points down away from the membrane. (H) Liposome-based SPR sensorgrams showing binding of CALM ANTH-WT, CALM ANTH(ΔH0), CALM ANTH(H0mut), and CALM ANTH(PIP−) to PtdIns4,5P2-containing liposomes at a protein concentration of 6 μM. (I) KD values for the binding of CALM ANTH domains, determined by liposome-based SPR (mean and SD of four independent measurements). Representative sensorgrams used for the measurements are shown in Figure S2A.
Figure 3
Structure of the CALM N-Terminal Amphipathic Helix (AH0) CALM ANTH domain is in gray, truncated CALM ANTH(1–264) in pale blue, and the CALM ANTH:VAMP8 complex in purple/green. (A–C) Three different structures of the first 289 residues of CALM with the binding site for PtdIns4,5P2 and the proposed position of the membrane indicated. AH0 is indicated with a dashed ellipse. (D and E) Positions (D) and ribbon representation (E) of α helices in the N-terminal 30 residues of the various structures. AH0 is indicated with a dashed rectangle. (F and G) Angled (F) and face on (G) ribbon representations of the N terminus of CALM ANTH(1–264), highlighting the positions of the key AH0 hydrophobic residues Leu6, Ile10, and Val17 mutated to serines.
Figure 4
The AH0 of the CALM ANTH Domain Senses Membrane Curvature and Promotes Membrane Deformation (A) Single liposome membrane curvature sensing assay showing CALM ANTHwt and CALM ANTH(ΔH0) density versus liposome diameter for PtdIns4,5P2 containing liposomes. Protein concentration = 500 nM. The marginal histograms (left) reveal the occurrence of protein densities for CALM ANTH-WT and CALM ANTH(ΔH0). (B) Tubulation of PtdIns4,5P2-containing liposomes by 0.5 μM CALM ANTH proteins for 1 min. Sample electron micrographs showing (Bi) no, (Bii) slight, and (Biii) extensive pearlized tubulation typically seen. Scale bar represents 500 nm. (C) CALM ANTH-WT causes extensive tubulation in ∼70% of micrographs, whereas CALM ANTH(ΔH0) CALM ANTH(H0mut) do so only in ∼2% and ∼3%, respectively. Error bars show the SEM.
Figure 5
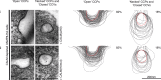
CALM with a Non-Functional AH0 Cannot Rescue CALM-Depleted CCP/CCV Profile Phenotypes The size variation of clathrin-coated structures, as a consequence of CALM depletion with re-expression of mutant versions of (i) CALM(ΔH0) and (ii) CALM(H0mut) compared to those found in control cells as indicated by the dotted red line. Left-hand columns show representative images of open and necked/closed clathrin-coated structures for each cell line. Right-hand columns show the traces of 100 randomly selected clathrin-coated structures and the percentage (∼150 images) of each type of structure adjacent to the traces. Scale bars represent 200 nm.
Figure 6
CALM’s AH0 Directly Affects Rates of Endocytosis (A and B) Internalization of (A) [125I] transferrin (Tf) and (B) [125I] EGF in HeLaM VAMP8-HA-expressing cells were measured for 2, 4, and 6 minutes. The amount of surface bound and internalized radioactivity was quantified and plotted against time. Each experiment was performed at least twice with triplicates for each time point. Error bars represent the SD of triplicates. Cells depleted of CALM showed an ∼50% reduction in endocytosis of both Tf and EGF compared to that of control cells. This endocytic defect is rescued with the expression of siRNA resistant CALMwt, but not with CALM(ΔH0). Cells depleted of AP2 showed an ∼90% reduction in endocytosis of both Tf and EGF compared to that of control cells. The bar chart (insert) shows a comparison of the internalization rate constants (ke), which represent the linear regression coefficients. Those for the internalization by control HeLaM VAMP8-HA cells were set to 100%; all other values were put in relation to it. Error bars represent the SD. (C and D) Immunofluorescence of cells after 6 min endocytosis of Tf-Alexa568 (C) or EGF-Alexa488 (D) by VAMP8-HA control and VAMP8-HA CALM-depleted cells. The images are maximum projections of ten focal z planes covering the whole volume of the imaged cells. Scale bar represents 15 μm.
Figure 7
AP2 Depletion and CALM Overexpression Alter CCP/CCV Profile Phenotypes Electron micrographs (left-hand columns) show a typical representation of an open and a necked/closed clathrin-coated structure for each cell line. Traces (right-hand columns) were made from 100 images and counts represented in percentages are of ∼150 images for each condition. Dotted red line represents average size of CCPs/CCVs in control cells. (A) The size variation of clathrin-coated structures are shown, and quantified AP2 (μ2 subunit)-depleted cells (Ai) show an obvious reduction in CCP/CCV size that can be rescued with the expression of an siRNA-resistant AP2-μ2 subunit (Aii). (B) The size variation of clathrin-coated structures as a consequence of expressing siRNA-resistant CALM variants in addition to endogenous CALM (without siRNA treatment) (Bii and Biii). The levels of CALM were approximately double that in control cells as shown by western blotting (Figure S1). Attempts to create cell lines expressing higher levels of CALM expression failed presumably because higher levels of CALM expression are toxic. (Bi) As before, control cells show a greater percentage of necked/closed clathrin-coated structures (∼70%) than open structures, and all profiles are within the size range previously obtained. Clathrin-coated structures in cells expressing endogenous and siRNA-resistant CALM-WT (Bii) were noticeably smaller than profiles of control profiles (note the size of the central cavity) (Bi) or of cells expressing endogenous and siRNA-resistant CALM(ΔH0) (Biii). In (Bii), there is also an increase in the percentage of necked/closed structures (85% as opposed to ∼70% for Bi and Biii). Scale bars represent 200 nm.
Comment in
- Zero tolerance: amphipathic helices in endocytosis.
Wood LA, Royle SJ. Wood LA, et al. Dev Cell. 2015 Apr 20;33(2):119-20. doi: 10.1016/j.devcel.2015.04.007. Dev Cell. 2015. PMID: 25898162
Similar articles
- Zero tolerance: amphipathic helices in endocytosis.
Wood LA, Royle SJ. Wood LA, et al. Dev Cell. 2015 Apr 20;33(2):119-20. doi: 10.1016/j.devcel.2015.04.007. Dev Cell. 2015. PMID: 25898162 - Uncoupling the functions of CALM in VAMP sorting and clathrin-coated pit formation.
Sahlender DA, Kozik P, Miller SE, Peden AA, Robinson MS. Sahlender DA, et al. PLoS One. 2013 May 31;8(5):e64514. doi: 10.1371/journal.pone.0064514. Print 2013. PLoS One. 2013. PMID: 23741335 Free PMC article. - Evolving models for assembling and shaping clathrin-coated pits.
Chen Z, Schmid SL. Chen Z, et al. J Cell Biol. 2020 Sep 7;219(9):e202005126. doi: 10.1083/jcb.202005126. J Cell Biol. 2020. PMID: 32770195 Free PMC article. Review. - Cargo recognition during clathrin-mediated endocytosis: a team effort.
Sorkin A. Sorkin A. Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
- Computational Modeling of Realistic Cell Membranes.
Marrink SJ, Corradi V, Souza PCT, Ingólfsson HI, Tieleman DP, Sansom MSP. Marrink SJ, et al. Chem Rev. 2019 May 8;119(9):6184-6226. doi: 10.1021/acs.chemrev.8b00460. Epub 2019 Jan 9. Chem Rev. 2019. PMID: 30623647 Free PMC article. Review. - FCHO controls AP2's initiating role in endocytosis through a PtdIns(4,5)P2-dependent switch.
Zaccai NR, Kadlecova Z, Dickson VK, Korobchevskaya K, Kamenicky J, Kovtun O, Umasankar PK, Wrobel AG, Kaufman JGG, Gray SR, Qu K, Evans PR, Fritzsche M, Sroubek F, Höning S, Briggs JAG, Kelly BT, Owen DJ, Traub LM. Zaccai NR, et al. Sci Adv. 2022 Apr 29;8(17):eabn2018. doi: 10.1126/sciadv.abn2018. Epub 2022 Apr 29. Sci Adv. 2022. PMID: 35486718 Free PMC article. - Molecularly Distinct Clathrin-Coated Pits Differentially Impact EGFR Fate and Signaling.
Pascolutti R, Algisi V, Conte A, Raimondi A, Pasham M, Upadhyayula S, Gaudin R, Maritzen T, Barbieri E, Caldieri G, Tordonato C, Confalonieri S, Freddi S, Malabarba MG, Maspero E, Polo S, Tacchetti C, Haucke V, Kirchhausen T, Di Fiore PP, Sigismund S. Pascolutti R, et al. Cell Rep. 2019 Jun 4;27(10):3049-3061.e6. doi: 10.1016/j.celrep.2019.05.017. Cell Rep. 2019. PMID: 31167147 Free PMC article. - Clathrin heavy chain phosphorylated at T606 plays a role in proper cell division.
Yabuno Y, Uchihashi T, Sasakura T, Shimizu H, Naito Y, Fukushima K, Ota K, Kogo M, Nojima H, Yabuta N. Yabuno Y, et al. Cell Cycle. 2019 Aug;18(16):1976-1994. doi: 10.1080/15384101.2019.1637201. Epub 2019 Jul 4. Cell Cycle. 2019. PMID: 31272276 Free PMC article. - Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission.
Schöneberg J, Lehmann M, Ullrich A, Posor Y, Lo WT, Lichtner G, Schmoranzer J, Haucke V, Noé F. Schöneberg J, et al. Nat Commun. 2017 Jun 19;8:15873. doi: 10.1038/ncomms15873. Nat Commun. 2017. PMID: 28627515 Free PMC article.
References
- Antonny B. Mechanisms of membrane curvature sensing. Annu. Rev. Biochem. 2011;80:101–123. - PubMed
- Bao H., Daniels R.W., MacLeod G.T., Charlton M.P., Atwood H.L., Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J. Neurophysiol. 2005;94:1888–1903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials