Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy - PubMed (original) (raw)
Review
. 2015 Feb 15;7(2):194-208.
eCollection 2015.
Affiliations
- PMID: 25901191
- PMCID: PMC4399086
Review
Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy
Li Duan et al. Am J Transl Res. 2015.
Abstract
Autologous chondrocyte implantation (ACI) is a golden treatment for large defects of the knee joint without osteoarthritis or other complications. Despite notable progresses, generation of a stable chondrocyte phenotype using progenitor cells remains a main obstacle for chondrocyte-based cartilage treatment. Monolayer chondrocyte expansion in vitro is accompanied by chondrocyte dedifferentiation, which produces a non-specific mechanically inferior extracellular matrix (ECM) unsuitable for ACI. In-depth understanding of the molecular events during chondrocyte dedifferentiation is required to maintain the capacity of in vitro expanded chondrocytes to produce hyaline cartilage-specific ECM. This review discusses key cytokines and signaling pathways involved in chondrocyte dedifferentiation from the standpoint of catabolism and anabolism. Some potential therapeutic strategies are also presented to counteract chondrocyte dedifferentiation for cell-based cartilage therapy.
Keywords: Chondrocyte dedifferentiation; autologous chondrocyte implantation; cartilage repair; cytokines; in vitro; signaling pathways.
Figures
Figure 1
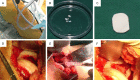
Autologous chondrocyte implantation. A. Cartilage biopsy was harvested from a non-weight bearing area of the knee joint under an arthoscope. B. Around 100 mg cartilage was harvested and enzymatically digested. Chondrocytes were propagated in a GMP lab. C. During the second operation, a collagen membrane was trimmed according to the defect size. D. The periosteum was sutured. E. Chondrocytes were injected into the space between the membrane and the defect. F. After chondrocyte injection, the membrane was sealed with fibrin glue to prevent leaking.
Figure 2
Chondrocyte dedifferentiation during in vitro expansion. Primary human chondrocyte morphology under invert microscope (×100). With passaging, round and polygonal chondrocytes were shifted to fibroblast-like cells. A (P0), B (P1), C (P2), D (P4).
Figure 3
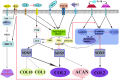
Schematic illustration of molecular events in chondrocyte dedifferentiation. Multiple signaling pathways are evoked during chondrocyte undergoing stimulants, including catabolic factors (IL-1β, IL-6, FGF-18) and anabolic factors (IGF-I, BMP-2, TGF-β). MAPK, PI3K/AKT, PKC, WNT, and Notch proteins are involved in regulating the ratio of COL-2 and COL-1, a key indicator of chondrocyte dedifferentiation. The red dash indicates inhibition and the black arrow indicates promotion.
Similar articles
- Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation.
Mao Y, Block T, Singh-Varma A, Sheldrake A, Leeth R, Griffey S, Kohn J. Mao Y, et al. Acta Biomater. 2019 Feb;85:75-83. doi: 10.1016/j.actbio.2018.12.006. Epub 2018 Dec 5. Acta Biomater. 2019. PMID: 30528605 - Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair.
Schulze-Tanzil G. Schulze-Tanzil G. Ann Anat. 2009 Oct;191(4):325-38. doi: 10.1016/j.aanat.2009.05.003. Epub 2009 Jun 6. Ann Anat. 2009. PMID: 19541465 Review. - Autologous chondrocyte implantation in the knee: systematic review and economic evaluation.
Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N. Mistry H, et al. Health Technol Assess. 2017 Feb;21(6):1-294. doi: 10.3310/hta21060. Health Technol Assess. 2017. PMID: 28244303 Free PMC article. Review. - Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction.
Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F, Mallein-Gerin F, Legendre F, Galera P. Demoor M, et al. Biochim Biophys Acta. 2014 Aug;1840(8):2414-40. doi: 10.1016/j.bbagen.2014.02.030. Epub 2014 Mar 6. Biochim Biophys Acta. 2014. PMID: 24608030 Review. - Overexpression of long non-coding RNA AP001505.9 inhibits human hyaline chondrocyte dedifferentiation.
Chen L, Xu J, Lv S, Zhao Y, Sun D, Zheng Y, Li X, Zhang L, Chi G, Li Y. Chen L, et al. Aging (Albany NY). 2021 Apr 4;13(8):11433-11454. doi: 10.18632/aging.202833. Epub 2021 Apr 4. Aging (Albany NY). 2021. PMID: 33839696 Free PMC article.
Cited by
- Novel insight on IRE1 in the regulation of chondrocyte dedifferentiation through ER stress independent pathway.
Eom YS, Shah FH, Kim SJ. Eom YS, et al. J Physiol Biochem. 2024 May;80(2):337-347. doi: 10.1007/s13105-024-01008-z. Epub 2024 Feb 10. J Physiol Biochem. 2024. PMID: 38336929 - Molecular Mechanisms in Pathophysiology of Mucopolysaccharidosis and Prospects for Innovative Therapy.
Ago Y, Rintz E, Musini KS, Ma Z, Tomatsu S. Ago Y, et al. Int J Mol Sci. 2024 Jan 17;25(2):1113. doi: 10.3390/ijms25021113. Int J Mol Sci. 2024. PMID: 38256186 Free PMC article. Review. - Activation of NOTCH1 by Shear Force Elicits Immediate Cytokine Expression in Human Chondrocytes.
Cheng HJ, Hsu WT, Chen CN, Li C. Cheng HJ, et al. Int J Mol Sci. 2020 Jul 14;21(14):4958. doi: 10.3390/ijms21144958. Int J Mol Sci. 2020. PMID: 32674293 Free PMC article. - Digoxin protects against intervertebral disc degeneration via TNF/NF-κB and LRP4 signaling.
Meng Q, Liu K, Liu Z, Liu J, Tian Z, Qin S, Wei J, Cheng L. Meng Q, et al. Front Immunol. 2023 Sep 18;14:1251517. doi: 10.3389/fimmu.2023.1251517. eCollection 2023. Front Immunol. 2023. PMID: 37790932 Free PMC article. - Managing the Heterogeneity of Mesenchymal Stem Cells for Cartilage Regenerative Therapy: A Review.
Goh D, Yang Y, Lee EH, Hui JHP, Yang Z. Goh D, et al. Bioengineering (Basel). 2023 Mar 13;10(3):355. doi: 10.3390/bioengineering10030355. Bioengineering (Basel). 2023. PMID: 36978745 Free PMC article. Review.
References
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. - PubMed
- Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, Uhl M, Ghanem N, Südkamp NP, Salzmann G. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42:150–157. - PubMed
- Malicev E, Kregar-Velikonja N, Barlic A, Alibegović A, Drobnic M. Comparison of articular and auricular cartilage as a cell source for the autologous chondrocyte implantation. J Orthop Res. 2009;27:943–948. - PubMed
- Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325–338. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources