Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication - PubMed (original) (raw)
Review
Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication
R Douglas Fields et al. Neuron. 2015.
Abstract
If "the connectome" represents a complete map of anatomical and functional connectivity in the brain, it should also include glia. Glia define and regulate both the brain's anatomical and functional connectivity over a broad range of length scales, spanning the whole brain to subcellular domains of synaptic interactions. This Perspective article examines glial interactions with the neuronal connectome (including long-range networks, local circuits, and individual synaptic connections) and highlights opportunities for future research. Our understanding of the structure and function of the neuronal connectome would be incomplete without an understanding of how all types of glia contribute to neuronal connectivity and function, from single synapses to circuits.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1
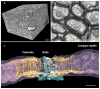
Conduction time between relay points in neural circuits must be precise for spike-timing dependent plasticity and sustaining oscillations over long-distance networks. Myelin is the most effective means of increasing conduction velocity; thus myelin strongly influences network function and by activity-dependent feedback, may contribute to nervous system plasticity. (A) Mouse optic nerve reconstructed from several hundred ultra-thin sections obtained by serial block-face electron microscopy. Such new methods are enabling network analysis of myelination at an ultrastructural level. (B) Optic nerve in cross section analyzed by transmission electron microscopy. Note the multiple layers of compact membrane (myelin) wrapped around axons. (C) Three-dimensional reconstruction of the node of Ranvier from serial block-face electron microscopy, as shown in A. Note the compact myelin (purple) wrapped around the axon (gray), forming a spiral channel of cytoplasm appearing as a series of paranodal loops flanking the node (gold). The electrogenic node of Ranvier, where voltage-gated sodium channels are concentrated, is ensheathed by perinodal astrocytes (blue). Scale bar = 10 um in A, 1 um in B and C.
Figure 2
Astrocytes are intimately associated with tens of thousands of synapses through highly ramified slender branches. Astrocytes can influence neuronal connectivity by binding multiple synapses and multiple neurons into functional assemblies, but astrocytes also operate at a subcellular level to sense and modulate synaptic activity at single synapses. (A) A single astrocyte from the neocortex of an adult mouse; note the cell body, multiple branches, and intricate fine highly-branched terminals. (B) An enlargement and surface rendering of the astrocyte processes shown in (A). Curtesy of Eric Bushong and Mark Ellisman at the National Center for Microscopy and Imaging Research, UCSD. See (Shigetomi et al., 2013) for additional information. The large tick marks are 5 μm in (A) and 0.5 um in (B).
Figure 3
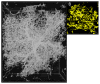
Microglia respond to nervous system damage but also monitor and remove synapses in an activity-dependent manner. (A) Microglia in a resting state in the CA1 area of hippocampus (green, Iba-1), nuclei are blue. (B) Activated microglia in vitro engulfing fluorescent-labeled latex beads. A, from Zhang et al., 2014. B, from Black and Waxman, 2014.
Similar articles
- Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity.
Fellin T. Fellin T. J Neurochem. 2009 Feb;108(3):533-44. doi: 10.1111/j.1471-4159.2008.05830.x. J Neurochem. 2009. PMID: 19187090 Review. - Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity.
Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR. Etxeberria A, et al. J Neurosci. 2016 Jun 29;36(26):6937-48. doi: 10.1523/JNEUROSCI.0908-16.2016. J Neurosci. 2016. PMID: 27358452 Free PMC article. - GLIA modulates synaptic transmission.
Perea G, Araque A. Perea G, et al. Brain Res Rev. 2010 May;63(1-2):93-102. doi: 10.1016/j.brainresrev.2009.10.005. Epub 2009 Nov 6. Brain Res Rev. 2010. PMID: 19896978 Review. - Plasticity of Neuron-Glial Transmission: Equipping Glia for Long-Term Integration of Network Activity.
Croft W, Dobson KL, Bellamy TC. Croft W, et al. Neural Plast. 2015;2015:765792. doi: 10.1155/2015/765792. Epub 2015 Aug 3. Neural Plast. 2015. PMID: 26339509 Free PMC article. Review. - Activity-dependent myelination: A glial mechanism of oscillatory self-organization in large-scale brain networks.
Noori R, Park D, Griffiths JD, Bells S, Frankland PW, Mabbott D, Lefebvre J. Noori R, et al. Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13227-13237. doi: 10.1073/pnas.1916646117. Epub 2020 Jun 1. Proc Natl Acad Sci U S A. 2020. PMID: 32482855 Free PMC article.
Cited by
- Role of stress-related glucocorticoid changes in astrocyte-oligodendrocyte interactions that regulate myelin production and maintenance.
Miguel-Hidalgo JJ. Miguel-Hidalgo JJ. Histol Histopathol. 2023 Jan;38(1):1-8. doi: 10.14670/HH-18-476. Epub 2022 Jun 2. Histol Histopathol. 2023. PMID: 35652516 Free PMC article. Review. - ANDA: an open-source tool for automated image analysis of in vitro neuronal cells.
Wæhler HA, Labba NA, Paulsen RE, Sandve GK, Eskeland R. Wæhler HA, et al. BMC Neurosci. 2023 Oct 24;24(1):56. doi: 10.1186/s12868-023-00826-z. BMC Neurosci. 2023. PMID: 37875799 Free PMC article. - Clinical relevance of terminal Schwann cells: An overlooked component of the neuromuscular junction.
Santosa KB, Keane AM, Jablonka-Shariff A, Vannucci B, Snyder-Warwick AK. Santosa KB, et al. J Neurosci Res. 2018 Jul;96(7):1125-1135. doi: 10.1002/jnr.24231. Epub 2018 Mar 13. J Neurosci Res. 2018. PMID: 29536564 Free PMC article. Review. - Zika virus encephalitis causes transient reduction of functional cortical connectivity.
Agner SC, Brier LM, Hill JD, Liu EY, Bice A, Rahn RM, Chen S, Culver JP, Klein RS. Agner SC, et al. Neurophotonics. 2025 Jan;12(Suppl 1):S14603. doi: 10.1117/1.NPh.12.S1.S14603. Epub 2024 Nov 28. Neurophotonics. 2025. PMID: 39610883 Free PMC article. - Shared and derived features of cellular diversity in the human cerebral cortex.
Miller DJ, Bhaduri A, Sestan N, Kriegstein A. Miller DJ, et al. Curr Opin Neurobiol. 2019 Jun;56:117-124. doi: 10.1016/j.conb.2018.12.005. Epub 2019 Jan 21. Curr Opin Neurobiol. 2019. PMID: 30677551 Free PMC article. Review.
References
- Avram AV, Basser PJ. Inferring millisecond-scale functional connectivity from tissue microstructure. Joint Annual Meeting ISMRM-ESMRAB; Milan, Italy. May 10–16; 2014. p. Abstract No. 6968.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources