Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA - PubMed (original) (raw)
. 2015 May 26;43(10):5120-9.
doi: 10.1093/nar/gkv415. Epub 2015 Apr 29.
Affiliations
- PMID: 25925567
- PMCID: PMC4446448
- DOI: 10.1093/nar/gkv415
Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA
Daan C Swarts et al. Nucleic Acids Res. 2015.
Abstract
Functions of prokaryotic Argonautes (pAgo) have long remained elusive. Recently, Argonautes of the bacteria Rhodobacter sphaeroides and Thermus thermophilus were demonstrated to be involved in host defense. The Argonaute of the archaeon Pyrococcus furiosus (PfAgo) belongs to a different branch in the phylogenetic tree, which is most closely related to that of RNA interference-mediating eukaryotic Argonautes. Here we describe a functional and mechanistic characterization of PfAgo. Like the bacterial counterparts, archaeal PfAgo contributes to host defense by interfering with the uptake of plasmid DNA. PfAgo utilizes small 5'-phosphorylated DNA guides to cleave both single stranded and double stranded DNA targets, and does not utilize RNA as guide or target. Thus, with respect to function and specificity, the archaeal PfAgo resembles bacterial Argonautes much more than eukaryotic Argonautes. These findings demonstrate that the role of Argonautes is conserved through the bacterial and archaeal domains of life and suggests that eukaryotic Argonautes are derived from DNA-guided DNA-interfering host defense systems.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
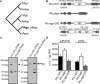
Figure 1.
_Pf_Ago interferes with plasmid transformation. (A) Schematic phylogenetic tree of Argonaute proteins, adapted from (3). _Af_Ago: Archaeoglobus fulgidus Ago. _Rs_Ago: Rhodobacter sphaeroides Ago. _Tt_Ago: Thermus thermophilus Ago. _Aa_Ago: Aquifex aeolicus Ago. _Pf_Ago: Pyrococcus furiosus Ago. Mj_Ago: Methanocaldococcus jannaschii Ago. eAgos: eukaryotic Agos. (B) Overview of ago gene loci of P. furiosus strains Pfu (wild-type), PfuΔ_ago (knockout) and Pfu-_ago_-O/E (_Pf_Ago overexpression strain). (C) Immunoblot analysis of Pf_Ago (indicated with a black triangle) content in Pfu and PfuΔ_ago with Csa2 protein (indicated with a gray triangle) serving as the internal standard (left panel) or Pfu and Pfu-_ago_-O/E (right panel). The amount of lysate analyzed is indicated and the asterisk (*) denotes apparent breakdown products observed when _Pf_Ago is overexpressed. (D) Plasmid transformation efficiencies of the P. furiosus strains. Error bars indicate standard deviations of biological triplicates.
Figure 2.
DEDH catalytic site of _Pf_Ago. (A) Sequence alignment of human AGO2 (hAGO2), _Tt_Ago, _Mj_Ago and _Pf_Ago, adapted from (3). Only regions containing the DEDX catalytic residues (indicated in red) are shown. _Pf_Ago catalytic residues E592 and E596 are colored orange. (B) _Tt_Ago catalytic residues DEDD (yellow; PDB: 4N47) aligned to _Pf_Ago catalytic site (black; PDB: 1Z25). Predicted catalytic residues of _Pf_Ago are colored green. (C) Synthetic 21 nucleotide siDNA (red) and 98 nucleotide ssDNA target (blue) used for in vitro activity assays. The black triangle indicates the predicted cleavage site, black lines indicate the predicted 59 and 39 nucleotide cleavage products. (D) _Pf_Ago and mutants were loaded with a 21 nucleotide long siDNA and were incubated with a 98 nucleotide ssDNA target in a 5:1:1 molar ratio (_Pf_Ago🦮target). Products were resolved on a 15% denaturing polyacrylamide gel. M: ssDNA marker. nt: nucleotide. The ‘Control’ sample contains no protein.
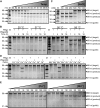
Figure 3.
Effect of temperature, salt concentration and cation on _Pf_Ago activity. _Pf_Ago loaded with a 21 nt long siDNA was incubated with a 98 nt ssDNA target (see Figure 2C) in a 5:1:1 molar ratio (_Pf_Ago🦮target) under various conditions. Unless otherwise indicated, target cleavage took place at 95°C for 1 h, with 0.5 mM Mn2+ as cation. Nucleic acids are resolved on denaturing polyacrylamide gels. M: ssDNA marker. nt: nucleotide. (A) _Pf_Ago activity is highest at temperatures between 90 and 99.9°C. (B) _Pf_Ago shows activity at temperatures ≥37°C if incubation is extended. (C) NaCl concentrations ≥500 mM interfere with _Pf_Ago activity. (D) _Pf_Ago-guide complexes show Co2+ and Mn2+ mediated ssDNA target cleavage. (E) Mn2+ is preferred above Co2+ as cation for _Pf_Ago-guide mediated ssDNA target cleavage.
Figure 4.
_Pf_Ago utilizes 15–31 nt long siDNAs for ssDNA target cleavage. _Pf_Ago was incubated with various guides and targets in a 5:1:1 ratio (_Pf_Ago🦮target). Unless otherwise indicated, target cleavage took place at 75°C for 1 h, with 0.5 mM Mn2+ as cation. Nucleic acids are resolved on denaturing polyacrylamide gels. M: ssDNA marker. (A) Synthetic DNA and RNA guides (black) and targets (gray). The black triangle indicates the predicted cleavage site, black lines indicate the predicted 11 and 34 nt cleavage products. (B) _Pf_Ago shows only DNA-guided DNA cleavage. (C and D) Both at 95 and 75°C, _Pf_Ago-mediated target cleavage is facilitated by siDNAs which are at least 15 nt long. C: control reaction with _Pf_AgoDM and 21 nt long siDNA.
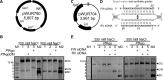
Figure 5.
Plasmid cleavage by _Pf_Ago. (A) pWUR790 expression plasmid. (B) _Pf_Ago expressed at 20°C and purified in absence of Mn2+ cleaves expression plasmid pWUR790. Agarose gels with plasmid targets incubated without protein (lane 1), with _Pf_AgoDM (lane 2) and with _Pf_Ago (lane 3). M1: 1-kb DNA ladder (New England Biolabs). M2: pWUR790 marker with open circular (OC), linearized (LIN) and supercoiled (SC) pWUR790. (C) pWUR704 target plasmid, target site indicated in gray. (D) Target region (gray) and FW and RV siDNA guides (black). Predicted cleavage sites are indicated with a black triangle. (E) Agarose gels with plasmid targets incubated with _Pf_AgoDM (lane 1), with guide free _Pf_Ago (lane 2) and with _Pf_Ago loaded with FW siDNA, RV siDNA, or both (lane 3–5) in reaction buffer with 250 mM NaCl (left panel) or 500 mM NaCl (right panel). M1: 1 kb GeneRuler marker (Thermo Scientific). M2: pWUR704 marker with open circular (OC), linearized (LIN) and supercoiled (SC) pWUR704.
Similar articles
- Molecular mechanism for target recognition, dimerization, and activation of Pyrococcus furiosus Argonaute.
Wang L, Chen W, Zhang C, Xie X, Huang F, Chen M, Mao W, Yu N, Wei Q, Ma L, Li Z. Wang L, et al. Mol Cell. 2024 Feb 15;84(4):675-686.e4. doi: 10.1016/j.molcel.2024.01.004. Epub 2024 Jan 30. Mol Cell. 2024. PMID: 38295801 - Rapid and cost-effective screening of CRISPR/Cas9-induced mutants by DNA-guided Argonaute nuclease.
Xiao G, Fu X, Zhang J, Liu S, Wang Z, Ye T, Zhang G. Xiao G, et al. Biotechnol Lett. 2021 Nov;43(11):2105-2110. doi: 10.1007/s10529-021-03177-z. Epub 2021 Sep 17. Biotechnol Lett. 2021. PMID: 34532823 Free PMC article. - The prokaryotic Argonaute proteins enhance homology sequence-directed recombination in bacteria.
Fu L, Xie C, Jin Z, Tu Z, Han L, Jin M, Xiang Y, Zhang A. Fu L, et al. Nucleic Acids Res. 2019 Apr 23;47(7):3568-3579. doi: 10.1093/nar/gkz040. Nucleic Acids Res. 2019. PMID: 30698806 Free PMC article. - DNA interference and beyond: structure and functions of prokaryotic Argonaute proteins.
Lisitskaya L, Aravin AA, Kulbachinskiy A. Lisitskaya L, et al. Nat Commun. 2018 Dec 4;9(1):5165. doi: 10.1038/s41467-018-07449-7. Nat Commun. 2018. PMID: 30514832 Free PMC article. Review. - A long look at short prokaryotic Argonautes.
Koopal B, Mutte SK, Swarts DC. Koopal B, et al. Trends Cell Biol. 2023 Jul;33(7):605-618. doi: 10.1016/j.tcb.2022.10.005. Epub 2022 Nov 22. Trends Cell Biol. 2023. PMID: 36428175 Review.
Cited by
- Mechanistic Insights into Archaeal and Human Argonaute Substrate Binding and Cleavage Properties.
Willkomm S, Zander A, Grohmann D, Restle T. Willkomm S, et al. PLoS One. 2016 Oct 14;11(10):e0164695. doi: 10.1371/journal.pone.0164695. eCollection 2016. PLoS One. 2016. PMID: 27741323 Free PMC article. - Evolutionary Ecology of Prokaryotic Immune Mechanisms.
van Houte S, Buckling A, Westra ER. van Houte S, et al. Microbiol Mol Biol Rev. 2016 Jul 13;80(3):745-63. doi: 10.1128/MMBR.00011-16. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27412881 Free PMC article. Review. - Characterization of a Programmable Argonaute Nuclease from the Mesophilic Bacterium Rummeliibacillus suwonensis.
Jiang X, Liu Y, Liu Q, Ma L. Jiang X, et al. Biomolecules. 2022 Feb 23;12(3):355. doi: 10.3390/biom12030355. Biomolecules. 2022. PMID: 35327547 Free PMC article. - Facilitated diffusion of Argonaute-mediated target search.
Cui TJ, Joo C. Cui TJ, et al. RNA Biol. 2019 Sep;16(9):1093-1107. doi: 10.1080/15476286.2019.1616353. Epub 2019 May 20. RNA Biol. 2019. PMID: 31068066 Free PMC article. Review. - Cytosolic Genomic DNA functions as a Natural Antisense.
Asada K, Ito K, Yui D, Tagaya H, Yokota T. Asada K, et al. Sci Rep. 2018 Jun 4;8(1):8551. doi: 10.1038/s41598-018-26487-1. Sci Rep. 2018. PMID: 29867148 Free PMC article.
References
- Ketting R.F. The many faces of RNAi. Dev. Cell. 2011;20:148–161. - PubMed
- Meister G. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 2013;14:447–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials