Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex - PubMed (original) (raw)
Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex
Adam T Brockett et al. PLoS One. 2015.
Abstract
Physical exercise enhances a wide range of cognitive functions in humans. Running-induced cognitive enhancement has also been demonstrated in rodents but with a strong emphasis on tasks that require the hippocampus. Additionally, studies designed to identify mechanisms that underlie cognitive enhancement with physical exercise have focused on running-induced changes in neurons with little attention paid to such changes in astrocytes. To further our understanding of how the brain changes with physical exercise, we investigated whether running alters performance on cognitive tasks that require the prefrontal cortex and whether any such changes are associated with astrocytic, as well as neuronal, plasticity. We found that running enhances performance on cognitive tasks known to rely on the prefrontal cortex. By contrast, we found no such improvement on a cognitive task known to rely on the perirhinal cortex. Moreover, we found that running enhances synaptic, dendritic and astrocytic measures in several brain regions involved in cognition but that changes in the latter measures were more specific to brain regions associated with cognitive improvements. These findings suggest that physical exercise induces widespread plasticity in both neuronal and nonneuronal elements and that both types of changes may be involved in running-induced cognitive enhancement.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
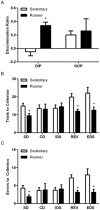
Fig 1. Running enhances cognitive performance on tasks known to require the medial prefrontal cortex and orbitofrontal cortex.
A, Running enhances performance on the object in place (OIP) task, but not on the novel object preference (NOP) task. B, Running results in fewer trials to criterion on the SD, REV and EDS. C, Running results in fewer errors on the SD, REV and EDS. Error bars represent SEM. *p<0.05 compared with sedentary rats for A-C. Complex discrimination (CD); intradimensional shift (IDS).
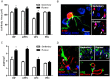
Fig 2. Running alters astrocyte morphology in regions associated with increased cognitive performance.
A, S100+ astrocyte cell body area is increased in the hippocampus, medial prefrontal cortex, and orbitofrontal cortex. B, Left: S100+ astrocyte (red) colabeled with GFAP (green). Scale bar = 5 μm. Right: Representative images of astrocytes from the medial prefrontal cortex of sedentary and running animals. Scale bar = 10 μm. C, Optical density of aquaporin-4, a water channel found in the endfeet of astrocytes, was increased in the hippocampus, medial prefrontal cortex, and orbitofrontal cortex of runners. D, Left: Aquaporin-4 (green) colabels with GFAP (red). Scale bar = 5 μm. Right top: Representative images of aquaporin-4 expression in CA1 of sedentary and running animals. Scale Bar = 20 μm. Right bottom: aquaporin-4 labeling (green) is shown in close proximity to smooth muscle actin labeling (red). Scale bar = 20 μm. Error bars represent SEM. *p<0.05 compared with Sedentary for A and C.
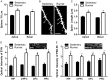
Fig 3. Running increases the number of dendritic spines in medial prefrontal cortex and expression of synaptic markers in several regions supporting cognitive function.
A, Running increases dendritic spine density on both apical and basal dendrites in the medial prefrontal cortex. B, Representative images of DiI labeled layer 2/3 pyramidal neuron apical dendrites in the medial prefrontal cortex and in sedentary and running animals. Scale Bar = 5 μm. C, Running increases the average length of spine processes. D, Optical intensity analysis of synaptophysin (SYN) reveals increased expression in all regions studied. Inset: example of synaptophysin staining in medial prefrontal cortex. E, PSD-95 levels are also increased in all regions studied. Inset: example of PSD-95 staining in medial prefrontal cortex. Scale Bar = 10 μm. Error bars represent SEM. *p<0.05 compared with sedentary for A, C-E.
Similar articles
- Sexual experience enhances cognitive flexibility and dendritic spine density in the medial prefrontal cortex.
Glasper ER, LaMarca EA, Bocarsly ME, Fasolino M, Opendak M, Gould E. Glasper ER, et al. Neurobiol Learn Mem. 2015 Nov;125:73-9. doi: 10.1016/j.nlm.2015.07.007. Epub 2015 Jul 15. Neurobiol Learn Mem. 2015. PMID: 26188276 - Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex.
Baudin A, Blot K, Verney C, Estevez L, Santamaria J, Gressens P, Giros B, Otani S, Daugé V, Naudon L. Baudin A, et al. Neurobiol Learn Mem. 2012 Oct;98(3):207-14. doi: 10.1016/j.nlm.2012.08.004. Epub 2012 Aug 24. Neurobiol Learn Mem. 2012. PMID: 22922490 - Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability.
Lapiz MD, Morilak DA. Lapiz MD, et al. Neuroscience. 2006 Feb;137(3):1039-49. doi: 10.1016/j.neuroscience.2005.09.031. Epub 2005 Nov 17. Neuroscience. 2006. PMID: 16298081 - Serotonin/dopamine interaction in memory formation.
González-Burgos I, Feria-Velasco A. González-Burgos I, et al. Prog Brain Res. 2008;172:603-23. doi: 10.1016/S0079-6123(08)00928-X. Prog Brain Res. 2008. PMID: 18772052 Review. - Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals.
Gruart A, Leal-Campanario R, López-Ramos JC, Delgado-García JM. Gruart A, et al. Neurobiol Learn Mem. 2015 Oct;124:3-18. doi: 10.1016/j.nlm.2015.04.006. Epub 2015 Apr 25. Neurobiol Learn Mem. 2015. PMID: 25916668 Review.
Cited by
- Treadmill Exercise Reverses the Change of Dendritic Morphology and Activates BNDF-mTOR Signaling Pathway in the Hippocampus and Cerebral Cortex of Ovariectomized Mice.
Feng Y, Tian X, Zhang M, Lou S. Feng Y, et al. J Mol Neurosci. 2021 Sep;71(9):1849-1862. doi: 10.1007/s12031-021-01848-0. Epub 2021 May 26. J Mol Neurosci. 2021. PMID: 34041687 - Moving fast, thinking fast: The relations of physical activity levels and bouts to neuroelectric indices of inhibitory control in preadolescents.
Pindus DM, Drollette ES, Raine LB, Kao SC, Khan N, Westfall DR, Hamill M, Shorin R, Calobrisi E, John D, Kramer AF, Hillman CH. Pindus DM, et al. J Sport Health Sci. 2019 Jul;8(4):301-314. doi: 10.1016/j.jshs.2019.02.003. Epub 2019 Feb 15. J Sport Health Sci. 2019. PMID: 31333883 Free PMC article. - Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies.
de Sousa Fernandes MS, Ordônio TF, Santos GCJ, Santos LER, Calazans CT, Gomes DA, Santos TM. de Sousa Fernandes MS, et al. Neural Plast. 2020 Dec 14;2020:8856621. doi: 10.1155/2020/8856621. eCollection 2020. Neural Plast. 2020. PMID: 33414823 Free PMC article. - Exercise Training-Related Changes in Cortical Gray Matter Diffusivity and Cognitive Function in Mild Cognitive Impairment and Healthy Older Adults.
Callow DD, Won J, Pena GS, Jordan LS, Arnold-Nedimala NA, Kommula Y, Nielson KA, Smith JC. Callow DD, et al. Front Aging Neurosci. 2021 Apr 8;13:645258. doi: 10.3389/fnagi.2021.645258. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33897407 Free PMC article. - Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice.
Niiranen L, Stenbäck V, Tulppo M, Herzig KH, Mäkelä KA. Niiranen L, et al. Int J Mol Sci. 2023 Feb 21;24(5):4259. doi: 10.3390/ijms24054259. Int J Mol Sci. 2023. PMID: 36901690 Free PMC article.
References
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11: 342–348. - PubMed
- Salis AS. Proactive and reactive effects of vigorous exercise on learning and vocabulary comprehension. Percept Mot Skills. 2013;116: 918–928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources