Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity - PubMed (original) (raw)
Review
Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity
Jolien Suurmond et al. J Clin Invest. 2015 Jun.
Abstract
In this Review we focus on the initiation of autoantibody production and autoantibody pathogenicity, with a special emphasis on the targeted antigens. Release of intracellular antigens due to excessive cell death or to ineffective clearance of apoptotic debris, modification of self-antigens during inflammatory responses, and molecular mimicry contribute to the initiation of autoantibody production. We hypothesize that those autoreactive B cells that survive and produce pathogenic autoantibodies have specificity for self-antigens that are TLR ligands. Such B cells experience both B cell receptor (BCR) activation and TLR engagement, leading to an escape from tolerance. Moreover, the autoantibodies they produce form immune complexes that can activate myeloid cells and thereby establish the proinflammatory milieu that further negates tolerance mechanisms of both B and T cells.
Figures
Figure 2. Proposed role of PRR activation and cross-reactivity in expansion of autoreactive B cells and their effector functions.
(A) Self-antigens that bind PRRs can activate B cells. We hypothesize that the combined recognition of self-antigens by BCRs and PRRs is required for tolerance escape in autoreactive B cells. (B) B cells require recognition of antigen through their BCR for clonal expansion. As most autoantibodies recognize intracellular self-antigens, cross-reactivity to cell surface or extracellular molecules enhances clonal expansion and differentiation into memory and plasma cells.
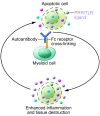
Figure 3. The role of PRR activation by self-antigens in autoantibody pathogenesis.
During autoimmune responses, inflammation can lead to cell death and release of endogenous PRR or TLR ligands. When recognized by autoantibodies, these ligands can activate myeloid immune cells through both Fc receptors and PRRs, leading to an enhanced inflammatory response. This response, in turn, can lead to tissue destruction and release of ligands for PRRs and autoantibodies, which further amplifies the chronic inflammatory response.
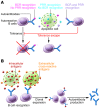
Figure 1. Mechanisms for autoantibody production: apoptosis, antigen modification, and cross-reactivity.
Three models can explain the recognition of intracellular antigens by autoantibodies. (A) Cell death through apoptosis or necrosis leads to extracellular exposure of intracellular self-antigens through release of intracellular contents into the extracellular environment, formation of apoptosis blebs, or NETosis. If clearance mechanisms are insufficient, there may be recognition of these antigens by B cells and autoantibody production. (B) Modification of self-antigen generates neoantigens, to which B cells have not been tolerized. (C) Autoantibody production arises from responses to foreign antigens, which cross-react with self-antigens.
Similar articles
- Autoantibodies Targeting Intracellular and Extracellular Proteins in Autoimmunity.
Burbelo PD, Iadarola MJ, Keller JM, Warner BM. Burbelo PD, et al. Front Immunol. 2021 Mar 8;12:548469. doi: 10.3389/fimmu.2021.548469. eCollection 2021. Front Immunol. 2021. PMID: 33763057 Free PMC article. Review. - Editorial: Lymphocytes reactive to autoantigens.
[No authors listed] [No authors listed] Lancet. 1973 Oct 27;2(7835):949-50. Lancet. 1973. PMID: 4126565 No abstract available. - Autoantibodies make a U-turn: the toll hypothesis for autoantibody specificity.
Martin DA, Elkon KB. Martin DA, et al. J Exp Med. 2005 Dec 5;202(11):1465-9. doi: 10.1084/jem.20052228. J Exp Med. 2005. PMID: 16330812 Free PMC article. Review. - The autoimmune diseases manifested by production of autoantibodies: the autoantigens identified by random peptide library.
Jadali Z, Sanati MH. Jadali Z, et al. Iran J Allergy Asthma Immunol. 2008 Sep;7(3):115-31. Iran J Allergy Asthma Immunol. 2008. PMID: 18780947 Review.
Cited by
- Detection of autoantibodies to heat shock protein 70 in the saliva and urine of normal individuals.
Sitko K, Mantej J, Bednarek M, Tukaj S. Sitko K, et al. Front Immunol. 2024 Jul 29;15:1454018. doi: 10.3389/fimmu.2024.1454018. eCollection 2024. Front Immunol. 2024. PMID: 39136018 Free PMC article. - A proteome-wide immuno-mass spectrometric identification of serum autoantibodies.
Music M, Soosaipillai A, Batruch I, Prassas I, Bogdanos DP, Diamandis EP. Music M, et al. Clin Proteomics. 2019 Jun 20;16:25. doi: 10.1186/s12014-019-9246-0. eCollection 2019. Clin Proteomics. 2019. PMID: 31249498 Free PMC article. - Analytical and clinical performance of different platforms simultaneously detecting 15 antinuclear antibodies.
Qin Y, Fan C, Wang Y, Feng M, Liang Z, Zhao X, Gao C, Luo J. Qin Y, et al. J Clin Lab Anal. 2022 Jul;36(7):e24554. doi: 10.1002/jcla.24554. Epub 2022 Jun 16. J Clin Lab Anal. 2022. PMID: 35708068 Free PMC article. - Relevance of Receptor for Advanced Glycation end Products (RAGE) in Murine Antibody-Mediated Autoimmune Diseases.
Eichhorst A, Daniel C, Rzepka R, Sehnert B, Nimmerjahn F, Voll RE, Chevalier N. Eichhorst A, et al. Int J Mol Sci. 2019 Jul 1;20(13):3234. doi: 10.3390/ijms20133234. Int J Mol Sci. 2019. PMID: 31266174 Free PMC article. - Secretion of autoimmune antibodies in the human subcutaneous adipose tissue.
Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Frasca D, et al. PLoS One. 2018 May 16;13(5):e0197472. doi: 10.1371/journal.pone.0197472. eCollection 2018. PLoS One. 2018. PMID: 29768501 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical