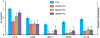Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility and Cellular Internalization of Paclitaxel: Preformulation and In vitro Assessments - PubMed (original) (raw)
Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility and Cellular Internalization of Paclitaxel: Preformulation and In vitro Assessments
Milin Shah et al. J Pharm Pharmacol (Los Angel). 2015.
Abstract
Drugs with low aqueous solubility and permeability possess substantial challenges in designing effective and safe formulations. Synergistic solubility and permeability enhancement in a simple formulation can increase bioavailability and efficacy of such drugs. To overcome limitations of the clinical formulation of Taxol®, Paclitaxel (PTX) was reformulated with various β-cyclodextrin (CD) derivatives suitable for parenteral administration. Results indicated that β-CDs can efficiently form complexes with PTX at lower molar ratios, enhance aqueous solubility up to 500 times and improved cellular internalization of PTX. All β-CD derivatives were found to be safe as excipient since none showed detectable signs of cyto-genotoxicity. As a result, the CD-PTX complexes significantly increased the cytotoxicity of the drug. The study concluded that CD-PTX formulations could substitute the current intravenous infusion of PTX obviating the use of non-inert excipient Cremophor EL.
Keywords: Compounds; Cyclodextrins; Enhancement; Inclusion; Permeability enhancement; Preformulation; Solubility.
Figures
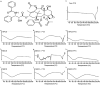
Figure 1
Representative differential scanning calorimetry (DSC) thermograms of free paclitaxel (PTX), _β_-cyclodextrins (CDs) and their mixtures and inclusion complexes with PTX; (a) – Chemical structure of PTX. (b) – Free PTX; (c) – Hydroxy propyl _β_-cyclodextrin (HPCD); (d) Physical mixture of HPCD and PTX (e) – Hydroxy propyl _β_-cyclodextrin-paclitaxel (HPCD-PTX); (f) – Methyl _β_-cyclodextrin (MeCD); (g) Physical mixture of MeCD and PTX; (h) – Methyl _β_-cyclodextrin-paclitaxel (MeCD-PTX); (i) – Sulfobutyl ether _β_-cyclodextrin (SBCD); (j) – Physical mixture of SBCD and PTX; (k) – Sulfobutyl ether _β_-cyclodextrin-paclitaxel (SBCD-PTX). A vertical bar on ordinate corresponds to 1 W/g of heat flow.
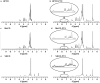
Figure 2
Representative nuclear magnetic resonance (NMR) spectra of _β_-cyclodextrins (CDs) and their inclusion complexes with paclitaxel (PTX). (a) – Hydroxy propyl _β_-cyclodextrin (HPCD); (b) – Hydroxy propyl _β_-cyclodextrin-paclitaxel (HPCD-PTX); (c) – Methyl _β_-cyclodextrin (MeCD); (d) – Methyl _β_-cyclodextrin-paclitaxel (MeCD-PTX); (e) – Sulfobutyl ether _β_-cyclodextrin (SBCD); (f) – Sulfobutyl ether _β_-cyclodextrin-paclitaxel (SBCD-PTX).
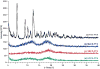
Figure 3
Representative X-ray diffraction spectra of free paclitaxel (PTX) and _β_-cyclodextrin (CD) inclusion complexes with PTX. (a) – Free PTX; (b) – Methyl _β_-cyclodextrin-paclitaxel (MeCD-PTX); (c) – Sulfobutyl ether _β_-cyclodextrin-paclitaxel (SBCD-PTX); (d) – Hydroxy propyl _β_-cyclodextrin-paclitaxel (HPCD-PTX).
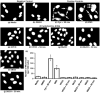
Figure 4
Genotoxicity of _β_-cyclodextrins (CDs). (a) – (j) – representative images of cells stained with nuclear dye incubated with the following substances: (a) – Media (untreated cells, negative control); (b) – DMSO (negative control for the solvent); (c) – Cyclophosphamide (Cyc) with metabolic activator – S9 mix (positive control 1); (d) – Ethyl methanesulfone – EMS (positive control 2); (e) – Hydroxy propyl _β_-cyclodextrin (HPCD); (f) – HPCD + S9; (g) – Methyl _β_-cyclodextrin (MeCD); (h) – MeCD + S9; (i) – Sulfobutyl ether _β_-cyclodextrin (SBCD); (j) – SBCD + S9. (k) – Quantitative analysis of micronuclei formation. Means ± SD are shown. *P < 0.05 when compared with control (untreated cells). Arrows indicate micronuclei.
Figure 5
Cytotoxicity of _β_-cyclodextrin (CD) – Paclitaxel (PTX) inclusion complexes in different cell lines. Specified cell types were incubated within 24 h with free PTX and CD-PTX complexes indicated. Means ± SD are shown. *P < 0.05 when compared with free PTX.
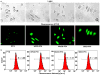
Figure 6
Cellular internalization of free and _β_-cyclodextrin – bound fluorescently labelled paclitaxel. (a) Representative light and fluorescence images of cells incubated within 3 h with substances indicated. (b) Representative flow cytometry fluorescence intensity charts. PTX – free non-bound paclitaxel; HPCD-PTX – Hydroxy propyl _β_-cyclodextrin-paclitaxel; MeCD-PTX – Methyl _β_-cyclodextrin-paclitaxel; SBCD-PTX – Sulfobutyl ether _β_-cyclodextrin-paclitaxel.
Similar articles
- A Promising Review on Cyclodextrin Conjugated Paclitaxel Nanoparticles for Cancer Treatment.
Velhal K, Barage S, Roy A, Lakkakula J, Yamgar R, Alqahtani MS, Yadav KK, Ahn Y, Jeon BH. Velhal K, et al. Polymers (Basel). 2022 Aug 3;14(15):3162. doi: 10.3390/polym14153162. Polymers (Basel). 2022. PMID: 35956677 Free PMC article. Review. - The preparation, characterization, and pharmacokinetic studies of chitosan nanoparticles loaded with paclitaxel/dimethyl-β-cyclodextrin inclusion complexes.
Ye YJ, Wang Y, Lou KY, Chen YZ, Chen R, Gao F. Ye YJ, et al. Int J Nanomedicine. 2015 Jul 3;10:4309-19. doi: 10.2147/IJN.S83508. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26170666 Free PMC article. - Ionic-Liquid-Based Paclitaxel Preparation: A New Potential Formulation for Cancer Treatment.
Chowdhury MR, Moshikur RM, Wakabayashi R, Tahara Y, Kamiya N, Moniruzzaman M, Goto M. Chowdhury MR, et al. Mol Pharm. 2018 Jun 4;15(6):2484-2488. doi: 10.1021/acs.molpharmaceut.8b00305. Epub 2018 May 18. Mol Pharm. 2018. PMID: 29762034 - Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluation.
Badr-Eldin SM, Elkheshen SA, Ghorab MM. Badr-Eldin SM, et al. Eur J Pharm Biopharm. 2008 Nov;70(3):819-27. doi: 10.1016/j.ejpb.2008.06.024. Epub 2008 Jul 4. Eur J Pharm Biopharm. 2008. PMID: 18655829 - Cyclodextrins: structure, physicochemical properties and pharmaceutical applications.
Jansook P, Ogawa N, Loftsson T. Jansook P, et al. Int J Pharm. 2018 Jan 15;535(1-2):272-284. doi: 10.1016/j.ijpharm.2017.11.018. Epub 2017 Nov 11. Int J Pharm. 2018. PMID: 29138045 Review.
Cited by
- Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation.
Di Donato C, Lavorgna M, Fattorusso R, Isernia C, Isidori M, Malgieri G, Piscitelli C, Russo C, Russo L, Iacovino R. Di Donato C, et al. Molecules. 2016 Dec 1;21(12):1644. doi: 10.3390/molecules21121644. Molecules. 2016. PMID: 27916966 Free PMC article. - Preparation and Properties of Cyclodextrin Inclusion Complexes of Hyperoside.
Zhang X, Su J, Wang X, Wang X, Liu R, Fu X, Li Y, Xue J, Li X, Zhang R, Chu X. Zhang X, et al. Molecules. 2022 Apr 25;27(9):2761. doi: 10.3390/molecules27092761. Molecules. 2022. PMID: 35566111 Free PMC article. - Injectable Glycol Chitosan Hydrogel Containing Folic Acid-Functionalized Cyclodextrin-Paclitaxel Complex for Breast Cancer Therapy.
Hyun H, Park MH, Jo G, Lee BY, Choi JW, Chun HJ, Kim HS, Yang DH. Hyun H, et al. Nanomaterials (Basel). 2021 Jan 27;11(2):317. doi: 10.3390/nano11020317. Nanomaterials (Basel). 2021. PMID: 33513732 Free PMC article. - Folate-appended cyclodextrin carrier targets ovarian cancer cells expressing the proton-coupled folate transporter.
Saito S, Koya Y, Kajiyama H, Yamashita M, Kikkawa F, Nawa A. Saito S, et al. Cancer Sci. 2020 May;111(5):1794-1804. doi: 10.1111/cas.14379. Epub 2020 Apr 3. Cancer Sci. 2020. PMID: 32154964 Free PMC article. - A Promising Review on Cyclodextrin Conjugated Paclitaxel Nanoparticles for Cancer Treatment.
Velhal K, Barage S, Roy A, Lakkakula J, Yamgar R, Alqahtani MS, Yadav KK, Ahn Y, Jeon BH. Velhal K, et al. Polymers (Basel). 2022 Aug 3;14(15):3162. doi: 10.3390/polym14153162. Polymers (Basel). 2022. PMID: 35956677 Free PMC article. Review.
References
- FDA. The biopharmaceutics classification system (BCS) guidance 2009
- Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. - PubMed
- Rodriguez-Antona C. Pharmacogenomics of paclitaxel. Pharmacogenomics. 2010;11:621–623. - PubMed
- Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. - PubMed
- Arbuck SG, Canetta R, Onetto N, Christian MC. Current dosage and schedule issues in the development of paclitaxel (Taxol) Semin Oncol. 1993;20:31–39. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials