IPS-1 differentially induces TRAIL, BCL2, BIRC3 and PRKCE in type I interferons-dependent and -independent anticancer activity - PubMed (original) (raw)
IPS-1 differentially induces TRAIL, BCL2, BIRC3 and PRKCE in type I interferons-dependent and -independent anticancer activity
S Kumar et al. Cell Death Dis. 2015.
Abstract
RIG-I-like receptors are the key cytosolic sensors for RNA viruses and induce the production of type I interferons (IFN) and pro-inflammatory cytokines through a sole adaptor IFN-β promoter stimulator-1 (IPS-1) (also known as Cardif, MAVS and VISA) in antiviral innate immunity. These sensors also have a pivotal role in anticancer activity through induction of apoptosis. However, the mechanism for their anticancer activity is poorly understood. Here, we show that anticancer vaccine adjuvant, PolyIC (primarily sensed by MDA5) and the oncolytic virus, Newcastle disease virus (NDV) (sensed by RIG-I), induce anticancer activity. The ectopic expression of IPS-1 into type I IFN-responsive and non-responsive cancer cells induces anticancer activity. PolyIC transfection and NDV infection upregulate pro-apoptotic gene TRAIL and downregulate the anti-apoptotic genes BCL2, BIRC3 and PRKCE. Furthermore, stable knockdown of IPS-1, IRF3 or IRF7 in IFN-non-responsive cancer cells show reduced anticancer activity by suppressing apoptosis via TRAIL and anti-apoptotic genes. Collectively, our study shows that IPS-1 induces anticancer activity through upregulation of pro-apoptotic gene TRAIL and downregulation of the anti-apoptotic genes BCL2, BIRC3 and PRKCE via IRF3 and IRF7 in type I IFN-dependent and -independent manners.
Figures
Figure 1
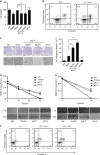
PolyIC transfection and NDV infection induce anticancer activity in various cancer cells. (a) HEK293T (b) MDAMB-231 cells were transfected with the indicated concentrations of polyIC (pIC-T) or stimulated with 25 _μ_g/ml of polyIC (pIC-S) along with the appropriate controls. After 24 h, the cell viability was determined using the MTT assay (a) or the cells were stained with API, and the level of apoptosis was determined using flow cytometry (b), 17AAG is a anticancer drug, used as a positive control. (c) Clonogenic assay: colonies of MDAMB-231 cells were pIC-T with the indicated concentrations or pIC-S with 25 _μ_g/ml, and the numbers of colonies in the plates were examined after 48 h. The left panel shows the microscopic images, and the right panel shows the numbers of dead cells or floating cells after different treatments. (d) IMR32 and (e) MDAMB-231 cell monolayers were pIC-T at 2 _μ_g/ml or pIC-S with 25 _μ_g/ml and wounds were scratched at 0 h. The upper panel shows the wound healing measured at 24, 48 and 72 h (d) or only at 24 h (e). The lower panel shows the wounds observed using light microscopy at 72 h (d) and 24 h (e). (f) MDAMB-231 cells were infected with the indicated MOI of NDV. After 24 h, the cells were stained with annexin V/PI, and apoptosis was determined by using flow cytometry
Figure 2
IPS-1 induces anticancer properties in various cancer cell lines. (a) HEK293T or (b) MDAMB-231 cells were transiently transfected with IPS-1, and 24 h later, the cell viability was determined using the MTT assay (a) or the cells were stained with annexin V/PI, and apoptosis was determined using flow cytometry (b). (c) IMR32 and (d) MDAMB-231 cell monolayers were transiently transfected with a FLAG-IPS-1 expression plasmid, and wounds were scratched at 0 h. The upper panel shows the wound healing measured at 24, 48 and 72 h (c) or only at 24 h (d). The bottom panel shows the wounds observed using light microscopy at 72 h (c) and 24 h (d). At the end of the experiment, the cell lysates were analyzed for the expression of IPS-1 by immunoblot analysis using an anti-FLAG-IPS-1 antibody (right). (e) FLAG-IPS-1 was transiently transfected in IMR32 and MDAMB-231 cells and cells supernatants were collected at indicated time-points to measure the level of IP-10 cytokine, and IPS-1 over-expression were confirmed by western blot using anti-FLAG antibody (right panel). (f) HEK293T cells were transiently transfected with the FLAG-IPS-1 plasmid. After 24 h, the cells were lysed to quantify the mRNA levels of apoptotic genes using qRT-PCR, and the over-expression of IPS-1 was confirmed by semi-quantitative PCR (below)
Figure 3
IPS-1 is required for anticancer activity. The efficacy of shRNA-mediated stable knockdown of IPS-1 in the MDAMB-231 cells (shIPS-A and -B) was validated by quantifying the protein levels of IPS-1 in the cells by (a) immunoblot and (b) confocal microscopic analysis using an anti-IPS-1 antibody. (c) shIPS-A and -B cells were transfected with polyIC, and 24 h later, the numbers of floating cells (dead cells) were counted (left) and images were taken (right). (d) shIPS-A and -B cells were grown until they formed colonies, which were transfected with polyIC. After 2 days, the numbers of colonies in the plates were examined using the clonogenic assay. (e) shIPS-A cells were transfected with polyIC. After 6 h, the mRNA levels of the indicated genes were quantified using qRT-PCR. The shIPS cells were infected with MOI=5 of NDV, and 24 h later, the mRNA levels of the NDV transcripts (f) and floating cells were counted and microscopic images were taken (g), or other genes as indicated were quantified using qRT-PCR (h). (i) MDAMB-231 cells were transiently transfected with a FLAG-IPS-1 expression plasmid. After 24 h, the cells were lysed, and the protein levels of the indicated genes were quantified by immunoblot (Cl., Cleaved)
Figure 4
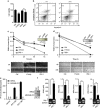
PolyIC transfection and IPS-1 over-expression induce the production of type I IFN, demonstrating anticancer activity. (a) IMR32 cells were transfected with polyIC, and 24 h later, the cells were lysed to quantify the mRNA level of various cytokines by qRT-PCR. (b) HEK293T and (c) IMR32 cells were transfected with IPS-1, C79I or C79F plasmids, and 24 h later, the levels of various cytokines quantified by qRT-PCR. IMR32 cell monolayers were transfected with IPS-1, C79I or C79F plasmids (d) or stimulated with indicated concentrations of recombinant IFN_β_ (f), and wounds were scratched at 0 h. The wound healing was measured at 24 h time intervals and observed using light microscopy. HEK293T cells were transfected with either IPS-1 or C79F (e) IMR32 cells were treated with rhuIFN_β_ (g) or transfected with IPS-1 plasmid (h). The mRNA level of TRAIL was quantified by qRT-PCR. Finally, the over-expression of IPS-1 or C79F was confirmed by semi-quantitative PCR or immunoblot analysis (lower panels)
Figure 5
IPS-1 induces type I IFN-independent anticancer activity. (a) MDAMB-231 and MCF10A cells were lysed and the expression levels of IFNAR_1 were quantified by qRT-PCR. (b) MCF10A and MDAMB-231 cells were transfected with polyIC. After three hours cells were lysed, and the mRNA levels of IFN_β, IP-10 and IRF3 were quantified by qRT-PCR. (c) MDAMB-231 and IMR32 cells were lysed and the expression levels of _IFNAR_1 were quantified by qRT-PCR. (d) MDAMB-231 and IMR32 cells were transfected with IPS-1 plasmid. After 36 h, the supernatants were collected, and the levels of the IP-10 cytokine were measured by ELISA. The over-expression of IPS-1 was confirmed by immunoblot analysis. (e) MDAMB-231 cells transiently transfected with FLAG-IPS-1 were subjected to the anchorage-independent colony-formation assay. (e) All cells were treated with the anticancer drug 17AAG (used as a positive control). At the end of experiment, the cell lysates were analyzed for the expression of IPS-1 by immunoblot analysis using an anti-FLAG-IPS-1 antibody (right). (f) MDAMB-231 cells were infected with pBABE or pBABE-IFNAR1 retrovirus and maintained for 48 h. These cells were seeded into 96-well plate followed by polyIC transfection or stimulation, after 30 h, cells viability assay was performed. MDAMB-231 and MCF10A cells were transfected with polyIC for 3 h (g) or NDV infection for 24 h (h). The cells were lysed and the mRNA levels of the indicated genes were quantified by using qRT-PCR
Figure 6
IPS-1 induces IRF3- and IRF7-dependent TRAIL to induce apoptosis in MDAMB-231 cells (a) HEK293T cells were transiently transfected with IRF3 or IRF7 expression plasmids, and 24 h later, the cells were lysed to quantify the mRNA levels of apoptotic genes by qRT-PCR. The over-expression of IRF3 and IRF7 was confirmed by semi-quantitative PCR (below). (b) MDAMB-231 cells were transiently transfected with IRF3/5D, after 24 h, cells were lysed and the mRNA level of p53 was quantified using qRT-PCR. (c) The MDAMB-231 cells were knocked down for IRF3 (shIRF3) or IRF7 (shIRF7) by specific shRNA and expression of IRF3 and IRF7 were analysed by semi-quantitative PCR, using GAPDH as the loading control. (d) Cells stably expressing shIRF3 or (e) shIRF7 cells were transfected with polyIC, and 24 h later, the numbers of floating cells were counted (left) and imaged by microscopy (right). (f) Cells stably expressing shIRF3 and shIRF7 cells were transfected with polyIC, and 3 h later, the mRNA levels of the indicated genes were measured by qRT-PCR. (g) shIRF3 cells were infected with NDV at an MOI of 5, and 24 h later, the numbers of floating cells were counted (left) and imaged by microscopy (right), and (h) the NDV transcripts, TRAIL, BCL2 and PRKCE mRNA levels were quantified by qRT-PCR
Figure 7
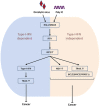
NDV infection and polyIC transfection induce type I IFN-dependent and -independent anticancer activity via the IPS-1, IRF3 and IRF7 axis. The mechanism of the type I IFN relies on high NDV replication, upregulation of TRAIL or downregulation of the BCL2, BIRC3 and PRKCE genes
Similar articles
- Pro-apoptotic signaling induced by Retinoic acid and dsRNA is under the control of Interferon Regulatory Factor-3 in breast cancer cells.
Bernardo AR, Cosgaya JM, Aranda A, Jiménez-Lara AM. Bernardo AR, et al. Apoptosis. 2017 Jul;22(7):920-932. doi: 10.1007/s10495-017-1377-z. Apoptosis. 2017. PMID: 28409399 - Phosphatidylinositol-3-kinase and Akt are required for RIG-I-mediated anti-viral signalling through cross-talk with IPS-1.
Yeon SH, Song MJ, Kang HR, Lee JY. Yeon SH, et al. Immunology. 2015 Feb;144(2):312-20. doi: 10.1111/imm.12373. Immunology. 2015. PMID: 25158146 Free PMC article. - Functional characterization of domains of IPS-1 using an inducible oligomerization system.
Takamatsu S, Onoguchi K, Onomoto K, Narita R, Takahasi K, Ishidate F, Fujiwara TK, Yoneyama M, Kato H, Fujita T. Takamatsu S, et al. PLoS One. 2013;8(1):e53578. doi: 10.1371/journal.pone.0053578. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308256 Free PMC article. - [Role of IPS-1 in type I IFN induction].
Kawai T, Akira S. Kawai T, et al. Nihon Rinsho. 2006 Jul;64(7):1231-5. Nihon Rinsho. 2006. PMID: 16841392 Review. Japanese. - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
- Exploring the biological behavior differences between retroperitoneal and non-retroperitoneal liposarcomas.
Xi Z, Zhuang A, Li X, Ming TM, Cheng Y, Zhang C, Xie F, Wang Y, Yan G, Zheng J, Lin Z, Zhang G, Li H, Wu T, He Q, Li W. Xi Z, et al. Heliyon. 2024 Jul 19;10(15):e34878. doi: 10.1016/j.heliyon.2024.e34878. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157358 Free PMC article. - Induction of apoptosis by double-stranded RNA was present in the last common ancestor of cnidarian and bilaterian animals.
Kozlovski I, Jaimes-Becerra A, Sharoni T, Lewandowska M, Karmi O, Moran Y. Kozlovski I, et al. PLoS Pathog. 2024 Jul 16;20(7):e1012320. doi: 10.1371/journal.ppat.1012320. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39012849 Free PMC article. - Immune responses elicited by ssRNA(-) oncolytic viruses in the host and in the tumor microenvironment.
Bykov Y, Dawodu G, Javaheri A, Garcia-Sastre A, Cuadrado-Castano S. Bykov Y, et al. J Cancer Metastasis Treat. 2023;9:10. doi: 10.20517/2394-4722.2022.92. Epub 2023 Apr 4. J Cancer Metastasis Treat. 2023. PMID: 37974615 Free PMC article. - ADAR1 has an oncogenic function and can be a prognostic factor in cervical cancer.
Nakamura K, Shigeyasu K, Okamoto K, Matsuoka H, Masuyama H. Nakamura K, et al. Sci Rep. 2023 Mar 23;13(1):4720. doi: 10.1038/s41598-023-30452-y. Sci Rep. 2023. PMID: 36959226 Free PMC article. - Influence of Butyrate on Impaired Gene Expression in Colon from Patients with High Blood Pressure.
Li J, Richards EM, Handberg EM, Pepine CJ, Alakrad E, Forsmark CE, Raizada MK. Li J, et al. Int J Mol Sci. 2023 Jan 31;24(3):2650. doi: 10.3390/ijms24032650. Int J Mol Sci. 2023. PMID: 36768972 Free PMC article.
References
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005; 6: 981–988. - PubMed
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005; 437: 1167–1172. - PubMed
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005; 122: 669–682. - PubMed
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 2005; 19: 727–740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous