In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin - PubMed (original) (raw)
In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin
Madhur D Shastri et al. PLoS One. 2015.
Abstract
Background: Enoxaparin, a mixture of anticoagulant and non-anticoagulant fractions, is widely used as an anticoagulant agent. However, it is also reported to possess anti-inflammatory properties. Our study indicated that enoxaparin inhibits the release of IL-6 and IL-8 from A549 pulmonary epithelial cells. Their release causes extensive lung tissue damage. The use of enoxaparin as an anti-inflammatory agent is hampered due to the risk of bleeding associated with its anticoagulant fractions. Therefore, we aimed to identify the fraction responsible for the observed anti-inflammatory effect of enoxaparin and to determine the relationship between its structure and biological activities.
Methods: A549 pulmonary epithelial cells were pre-treated in the presence of enoxaparin and its fractions. The levels of IL-6 and IL-8 released from the trypsin-stimulated cells were measured by ELISA. The anticoagulant activity of the fraction responsible for the effect of enoxaparin was determined using an anti-factor-Xa assay. The fraction was structurally characterised using nuclear magnetic resonance. The fraction was 2-O, 6-O or N-desulfated to determine the position of sulfate groups required for the inhibition of interleukins. High-performance size-exclusion chromatography was performed to rule out that the observed effect was due to the interaction between the fraction and trypsin or interleukins.
Results: Enoxaparin (60 μg/mL) inhibited the release of IL-6 and IL-8 by >30%. The fraction responsible for this effect of enoxaparin was found to be a disaccharide composed of α-L-iduronic-acid and α-D-glucosamine-6-sulfate. It (15 μg/mL) inhibited the release of interleukins by >70%. The 6-O sulphate groups were responsible for its anti-inflammatory effect. The fraction did not bind to trypsin or interleukins, suggesting the effect was not due to an artefact of the experimental model.
Conclusion: The identified disaccharide has no anticoagulant activity and therefore eliminates the risk of bleeding associated with enoxaparin. Future in-vivo studies should be designed to validate findings of the current study.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
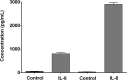
Fig 1. In-vitro interleukin release.
Trypsin-induced release of IL-6 and IL-8 from the epithelial cell culture supernatants. Data is presented as mean ± SD.
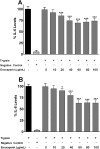
Fig 2. Concentration-dependent effect of enoxaparin on interleukin release.
Effect of different concentration of enoxaparin on the release of IL-6 (A) and IL-8 (B) following trypsin-induced in-vitro stimulation epithelial cells. Data is presented as percentage (mean ± SD) of the maximal observed IL-6 and IL-8 concentrations. *p<0.05, and ***p<0.001 versus trypsin-stimulated control.
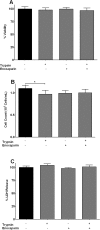
Fig 3. Effect of enoxaparin on cellular viability and proliferation.
(A) Viability of epithelial cells was determined by trypan blue dye exclusion test and is presented as % of viable cells remaining after 24 hours of incubation with trypsin, enoxaparin or trypsin + enoxaparin. Data is presented as mean ± SD. (B) Proliferation of epithelial cells was determined after 24 hours in the presence of trypsin, enoxaparin or trypsin + enoxaparin. Cells were counted after incubation for 24 hours and expressed as 105 cells/mL. Data is presented as mean ± SD. *p<0.05 versus unstimulated control. (C) Viability of epithelial cells is presented as mean % LDH release ± SD. Cells were incubated for 24 hours in the presence of trypsin, enoxaparin or trypsin + enoxaparin.
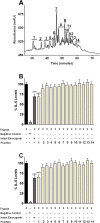
Fig 4. Effect of enoxaparin fractions on interleukin release.
Ion-exchange chromatographic (IC) separation of enoxaparin. The separations were performed using a CarboPac PA100 semi-preparative column; optimised NaCl gradient was from 32–74% over 70 minutes with a flow rate of 2 mL/minute and UV detection wavelength of 232 nm. The numbers indicate the area of all the fractions collected. Data represents a typical experiment out of ten independent experiments (A). Inhibition of IL-6 (B) and IL-8 (C) release by IC-derived 14 fractions of enoxaparin (60 μg/mL) after trypsin-induced in-vitro stimulation of epithelial cells. The relative percentile amount of each fraction (fraction 1 to 14) present in 60 μg/mL of intact enoxaparin was: 9.18%, 4.24%, 6.12%, 5.15%, 2.99%, 4.78%, 20.32%, 11.4%, 10.53%, 7.35%, 5.98%, 4.6%, 2.95% and 5.06% respectively. Data is presented as percentage (mean ± SD) of the maximal observed IL-6 and IL-8 concentrations. *p<0.05, and ***p<0.001 versus trypsin-stimulated control.
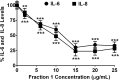
Fig 5. Concentration-dependent effect of IC-derived fraction 1 on interleukin release.
Inhibition of IL-6 and IL-8 release by fraction 1 of enoxaparin (0 to 25 μg/mL) after in-vitro stimulation of epithelial cells via trypsin. Data is presented as percentage of trypsin-stimulated control (mean ± SD). **p<0.01 and ***p<0.001 versus trypsin-stimulated control.
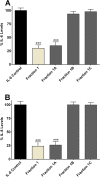
Fig 6. Effect of sub-fractions of IC-derived fraction 1 on interleukin release.
Inhibition of IL-6 (A) and IL-8 (B) release by enoxaparin fraction 1 and its three different sub-fractions (1A, 1B and 1C) after trypsin-induced in-vitro stimulation of epithelial cells. Data is presented as percentage (mean ± SD) of the maximal observed IL-6 and IL-8 concentrations. ***p<0.001 versus trypsin-stimulated control.
Fig 7. 13C-1H HSQC multiplicity edited spectrum for enoxaparin sub-fraction 1A.
Axis units are chemical shifts in parts per million (ppm). The dark blue contours denote correlations between carbon atoms with one or three attached protons and cyan contours carbons with a single attached proton. The atom nomenclature from Table 1 is applied to the proton and carbon axis projections.
Fig 8. 13C-1H HSQC-TOCSY 120ms spectrum for enoxaparin sub-fraction 1A.
Axis units are chemical shifts in parts per million (ppm). The atom nomenclature from Table 1 is applied to the proton and carbon axis projections.
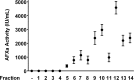
Fig 9. Anti-factor Xa activity of fourteen enoxaparin fractions derived using IC technique.
The anti-factor Xa assay is described in the experimental section. Data is presented as mean ± SD (n = 3).
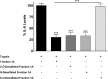
Fig 10. Effect of various desulfated fraction 1A on IL-6 release.
Epithelial cells were stimulated with trypsin in the presence of either 2-_O_-desulfated, _N_-desulfated or 6-_O_-desulfated fraction 1A of enoxaparin. Data is presented as mean ± SD. ***p<0.001 versus trypsin-stimulated control.
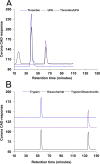
Fig 11. Binding of proteins with disaccharide of enoxaparin.
Interaction of UFH with thrombin. HP-SEC chromatograms of UFH (10 μM), thrombin (10 μM) and a mixture of UFH/thrombin (1:1 molar ratio) (A). Interaction of IC-derived disaccharide with trypsin. HP-SEC chromatograms of enoxaparin disaccharide (500 μM), trypsin (10 μM) and a mixture of trypsin/disaccharide (1:50 molar ratio) (B). Details of the HP-SEC experimental conditions are given in Methods.
Similar articles
- Therapeutic Potential of Enoxaparin in Lichen Planus: Exploring Reasons for Inconsistent Reports.
Patel RP, Shastri MD, Ming LC, Zaidi STR, Peterson GM. Patel RP, et al. Front Pharmacol. 2018 Jun 5;9:586. doi: 10.3389/fphar.2018.00586. eCollection 2018. Front Pharmacol. 2018. PMID: 29922163 Free PMC article. Review. - Non-Anticoagulant Fractions of Enoxaparin Suppress Inflammatory Cytokine Release from Peripheral Blood Mononuclear Cells of Allergic Asthmatic Individuals.
Shastri MD, Stewart N, Horne J, Zaidi ST, Sohal SS, Peterson GM, Korner H, Gueven N, Patel RP. Shastri MD, et al. PLoS One. 2015 Jun 5;10(6):e0128803. doi: 10.1371/journal.pone.0128803. eCollection 2015. PLoS One. 2015. PMID: 26046354 Free PMC article. - Opposing effects of low molecular weight heparins on the release of inflammatory cytokines from peripheral blood mononuclear cells of asthmatics.
Shastri MD, Stewart N, Eapen M, Peterson GM, Zaidi ST, Gueven N, Sohal SS, Patel RP. Shastri MD, et al. PLoS One. 2015 Mar 4;10(3):e0118798. doi: 10.1371/journal.pone.0118798. eCollection 2015. PLoS One. 2015. PMID: 25738575 Free PMC article. - Ion exchange chromatographic separation and isolation of oligosaccharides of intact low-molecular-weight heparin for the determination of their anticoagulant and anti-inflammatory properties.
Shastri MD, Johns C, Hutchinson JP, Khandagale M, Patel RP. Shastri MD, et al. Anal Bioanal Chem. 2013 Jul;405(18):6043-52. doi: 10.1007/s00216-013-6996-9. Epub 2013 May 28. Anal Bioanal Chem. 2013. PMID: 23712644 - Asthma drugs counter-regulate interleukin-8 release stimulated by sodium sulfite in an A549 cell line.
Yang YF, Hsu JY, Fu LS, Weng YS, Chu JJ. Yang YF, et al. J Asthma. 2009 Apr;46(3):238-43. doi: 10.1080/02770900802628508. J Asthma. 2009. PMID: 19373630
Cited by
- COVID-19 and Hypercoagulability: A Review.
Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J, Wani F, Kumar A, Vallabhaneni S, Khan MZ. Kichloo A, et al. Clin Appl Thromb Hemost. 2020 Jan-Dec;26:1076029620962853. doi: 10.1177/1076029620962853. Clin Appl Thromb Hemost. 2020. PMID: 33074732 Free PMC article. Review. - Antithrombotic and Anti-Inflammatory Effects of Fondaparinux and Enoxaparin in Hospitalized COVID-19 Patients: The FONDENOXAVID Study.
Cardillo G, Viggiano GV, Russo V, Mangiacapra S, Cavalli A, Castaldo G, Agrusta F, Bellizzi A, Amitrano M, Iannuzzo M, Sacco C, Lodigiani C, Fontanella A, Di Micco P; FondenoxavidStudy Group. Cardillo G, et al. J Blood Med. 2021 Feb 11;12:69-75. doi: 10.2147/JBM.S285214. eCollection 2021. J Blood Med. 2021. PMID: 33603528 Free PMC article. - Therapeutic Potential of Enoxaparin in Lichen Planus: Exploring Reasons for Inconsistent Reports.
Patel RP, Shastri MD, Ming LC, Zaidi STR, Peterson GM. Patel RP, et al. Front Pharmacol. 2018 Jun 5;9:586. doi: 10.3389/fphar.2018.00586. eCollection 2018. Front Pharmacol. 2018. PMID: 29922163 Free PMC article. Review. - Production of Dermatophagoides farinae Having Low Bacterial Content Using Ampicillin.
Kim JY, Yi MH, Kim M, Choi JH, Lee S, Yong TS. Kim JY, et al. J Immunol Res. 2023 May 18;2023:9024595. doi: 10.1155/2023/9024595. eCollection 2023. J Immunol Res. 2023. PMID: 37252681 Free PMC article. - The Use of the Anticoagulant Heparin and Corticosteroid Dexamethasone as Prominent Treatments for COVID-19.
Braz-de-Melo HA, Faria SS, Pasquarelli-do-Nascimento G, Santos IO, Kobinger GP, Magalhães KG. Braz-de-Melo HA, et al. Front Med (Lausanne). 2021 Apr 23;8:615333. doi: 10.3389/fmed.2021.615333. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33968948 Free PMC article. Review.
References
- Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89: 1001–1009. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources