Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein - PubMed (original) (raw)
Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein
M R Tanas et al. Oncogene. 2016.
Abstract
The WWTR1 (protein is known as TAZ)-CAMTA1 (WC) fusion gene defines epithelioid hemangioendothelioma, a malignant vascular cancer. TAZ (transcriptional coactivator with PDZ binding motif) is a transcriptional coactivator and end effector of the Hippo tumor suppressor pathway. It is inhibited by phosphorylation by the Hippo kinases LATS1 and LATS2. Such phosphorylation causes cytoplasmic localization, 14-3-3 protein binding and the phorphorylation of a terminal phosphodegron promotes ubiquitin-dependent degradation (the phosphorylation of the different motifs has several effects). CAMTA1 is a putative tumor suppressive transcription factor. Here we demonstrate that TAZ-CAMTA1 (TC) fusion results in its nuclear localization and constitutive activation. Consequently, cells expressing TC display a TAZ-like transcriptional program that causes resistance to anoikis and oncogenic transformation. Our findings elucidate the mechanistic basis of TC oncogenic properties, highlight that TC is an important model to understand how the Hippo pathway can be inhibited in cancer, and provide approaches for targeting this chimeric protein.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
Figure 1
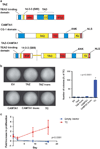
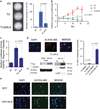
TC structure and oncogenic effects in vitro (a) Structure of full-length TAZ, CAMTA1, and the TC fusion protein. TAZ is 400 amino acids in length (44Kd) and contains a TEAD binding domain, a 14-3-3 protein binding motif, a WW domain, a transactivation domain including a coiled coil domain and a PDZ protein binding motif. CAMTA1 is 1673 amino acids in length (184Kd) and contains a CG-1 domain thought to bind CG-rich DNA sequences, a transactivation domain (TAD), a transcription factor immunoglobulin (TIG) domain, a series of ankyrin repeats (ANK) and three IQ calmodulin-binding motifs (IQ refers to the first two amino acids of the motif, isoleucine (I) and glutamine (Q)). While there is some microheterogeneity between different WWTR1_–_CAMTA1 gene fusions found in different EHEs, the most inclusive TAZ-CAMTA1 fusion protein is 1594 AA’s in length and predicts a 173Kd protein. The TAZ-CAMTA1 fusion protein is composed of the TEAD binding domain, 14-3-3 protein binding motif, and WW domain donated from TAZ fused in frame to the TAD TIG domain, ankyrin repeats, IQ domain and nuclear localization signal (NLS). S51 is critical for binding to TEAD proteins. (b) Soft agar assay with NIH/3T3 cells expressing empty vector, the TC fusion protein, full-length TAZ, full-length CAMTA1, as well as the truncated portions of TAZ and CAMTA1 involved in the fusion protein. Quantitative results graphically represented on the right. (c) Proliferation assay of cells to grow in suspension. NIH/3T3 cells expressing TC, but not TAZ or EV were able to grow in suspension. Error bars (b and c) represent one s.d.; experiments were performed at least twice. EV= empty vector.
Figure 2
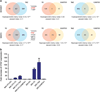
TC promotes a predominantly TAZ-like gene expression signature (a) Diagrammatic representation of the overlap of differentially expressed genes between TAZ-CAMTA1 (TC), TAZ and CAMTA1 in HEK293 and NIH/3T3 cells. (b) Quantitative RT-PCR expression of CTGF in HEK293 and NIH/3T3 derivative cell lines. Error bars (b) represent one s.d.; values are averages of three separate wells from a single experiment. Values are normalized to EV. EV=empty vector.
Figure 3
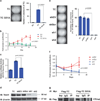
TC binds DNA through interaction with Tead4. Substitution of serine 51 for alanine (S51A) in TC resulted in abrogation of colony formation in soft agar (a) and decreased proliferation (b) in suspension. (c) TC S51A showed significantly reduced transcriptional activity by a Tead4 binding site luciferase reporter assay in TC S51A-HEK293 cells. (d) Effective RNA interference silencing of Tead4 in TC-NIH/3T3 cells. TC (TC-expressing cells), shEV (short hairpin empty vector control), shScr (scrambled empty vector control), sh1 (short hairpin Tead4 knock-down 1), sh2 (short hairpin Tead4 knock-down construct 2). (e) Silencing of Tead4 resulted in abrogation of colony formation in soft agar with sh1 and sh2 in TC-NIH/3T3 cells. (f) Silencing of Tead4 inhibited the ability of TC-NIH/3T3 cells to grow in suspension. (g) TC-NIH/3T3 cells and TCS51A-NIH/3T3 cells were transfected with Myc-Tead4, immunoprecipitated with anti-Myc antibody, then probed with anti-Flag antibody, showing that TC co-IPs with TEAD4 whereas TCS51A does not. Error bars (B, C, F) represent one s.d.; all experiments performed at least twice.
Figure 4
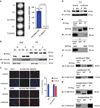
TC is constitutively active and not regulated by the Hippo pathway. (a) Expression of TAZ promotes minimal colony formation in NIH/3T3 cells in soft agar, whereas expression of TAZ S89A in NIH/3T3 cells shows a marked increase in the number of colonies formed in soft agar, showing that TAZ is negatively regulated by the Hippo pathway. Expression of TCS89A in NIH/3T3 cells does not result in an increase of colonies when compared with expression of TC in NIH/3T3 cells, indicating that TC is not regulated by Hippo. (b) In NIH/3T3 cells grown in suspension, TAZ translocates out of the nucleus into the cytoplasm, consistent with its regulation by the Hippo pathway. When detached, the nuclear to cytoplasmic equilibrium of TC does not change; further supporting that TC is not regulated by Hippo. (c) Immunofluorescence (IF) reveals TAZ to be located within the nucleus of NIH/3T3 cells when they are plated sparsely. When they are plated at confluent conditions, the Hippo pathway is activated and the TAZ signal is absent because TAZ translocates into the cytoplasm and is subsequently degraded. TC is located within the nucleus during both sparse and confluent conditions, indicating that it is no longer regulated by the Hippo pathway. Scale bar=20 microns. (d) Western blot shows that when TAZ-NIH/3T3 cells are plated under confluent conditions, addition of MG132 (MG) rescues TAZ levels to those found under sparse conditions with DMSO. This confirms that TAZ is degraded under confluent conditions and explains the lack of TAZ signal seen with IF under confluent conditions. (e) Co-immunoprecipitation experiments - Flag-TAZ and Flag-TC were pulled down with anti-Flag antibody and probed with 14-3-3ε antibody. Whereas flag-TAZ coimmunoprecipitates with 14-3-3ε, flag-TC does not, providing a putative mechanism for TC escape from Hippo pathway regulation. Evaluation of S89 phosphorylation during confluent conditions (f) and when grown in suspension (g). Immunoprecipitation with anti-Flag antibody is done first to eliminate non-specific bands with the phospho-S127 YAP antibody. Both TAZ and TC are phosphorylated on serine 89 (homologous to S127 in YAP) during confluent conditions and while grown in suspension. Serine 89 on TC is phosphorylated even though Hippo functionally is not regulating TC. Error bars (a, b, c) represent one s.d.; all experiments performed at least twice.
Figure 5
CAMTA1 contributes a NLS to TC. (a) NIH/3T3 cells expressing the TCΔNLS construct (lacking the putative NLS) demonstrate a markedly reduced ability to form colonies in soft agar. (b) TCΔNLS expressing cells display a reduced ability to proliferate in suspension compared with TC. (c) TCΔNLS-NIH/3T3 cells show a reduced ability to stimulate transcription in a Tead4 luciferase reporter assay. (d) TCΔNLS is rarely expressed in the nuclear compartment of NIH/3T3 cells. MG132 treatment results in TCΔNLS accumulation within the cytoplasm, indicating that without a NLS, the equilibrium of TCΔNLS is shifted to the cytoplasm where it is subsequently degraded. (d) In whole cell lysates, TCΔNLS expression is negligible. With MG132 treatment, TCΔNLS can be detected supporting the idea that it is retained in the cytoplasm and degraded. Nuclear and cytoplasmic fractionation of TCΔNLS treated with MG132 shows that TCΔNLS is localized predominantly within the cytoplasm of cells, consistent with the IF findings. (e) Immunofluoresence reveals that GFP is localized predominantly to the cytoplasm but also to the nucleus (top row) while GFP-NLS (bottom row) is localized exclusively to the nucleus. Error bars (a, b, c) represent one s.d.; all experiments performed at least twice.
Figure 6
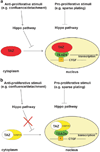
Working model of TC function. (a) In cellular contexts which favor proliferation (for example, sparse plating or developing organs), the Hippo pathway is inhibited, leading to TAZ localization within the nucleus where it complexes with TEAD4 and activates its transcriptional program. Importantly, TAZ is negatively regulated by the Hippo pathway in cell contexts where the Hippo pathway is activated (confluence/detachment). (b) During cellular contexts where the Hippo pathway is inactivated (for example, sparse plating or in organs of normal size), TC functions in the same way as TAZ and is located in the nucleus, where it activates a TAZ-like transcriptional program, including the activation of canonical TAZ targets such as CTGF. In contrast to TAZ, WC is no longer regulated by the Hippo pathway, and, utilizing a NLS from the C-terminus of CAMTA1, is constitutively located within the nucleus even in conditions where the Hippo pathway is activated (confluence/detachment).
Similar articles
- WWTR1(TAZ)-CAMTA1 reprograms endothelial cells to drive epithelioid hemangioendothelioma.
Driskill JH, Zheng Y, Wu BK, Wang L, Cai J, Rakheja D, Dellinger M, Pan D. Driskill JH, et al. Genes Dev. 2021 Apr 1;35(7-8):495-511. doi: 10.1101/gad.348221.120. Epub 2021 Mar 25. Genes Dev. 2021. PMID: 33766984 Free PMC article. - A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites.
Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. Errani C, et al. Genes Chromosomes Cancer. 2011 Aug;50(8):644-53. doi: 10.1002/gcc.20886. Epub 2011 May 16. Genes Chromosomes Cancer. 2011. PMID: 21584898 Free PMC article. - TAZ-CAMTA1 and YAP-TFE3 alter the TAZ/YAP transcriptome by recruiting the ATAC histone acetyltransferase complex.
Merritt N, Garcia K, Rajendran D, Lin ZY, Zhang X, Mitchell KA, Borcherding N, Fullenkamp C, Chimenti MS, Gingras AC, Harvey KF, Tanas MR. Merritt N, et al. Elife. 2021 Apr 29;10:e62857. doi: 10.7554/eLife.62857. Elife. 2021. PMID: 33913810 Free PMC article. - Pleural epithelioid hemangioendothelioma harboring CAMTA1 rearrangement.
Ha SY, Choi IH, Han J, Choi YL, Cho JH, Lee KJ, Sun JM. Ha SY, et al. Lung Cancer. 2014 Mar;83(3):411-5. doi: 10.1016/j.lungcan.2013.12.015. Epub 2014 Jan 7. Lung Cancer. 2014. PMID: 24461304 Review. - Epithelioid hemangioendothelioma-its history, clinical features, molecular biology and current therapy.
Tsuchihashi K, Baba E. Tsuchihashi K, et al. Jpn J Clin Oncol. 2024 Jul 7;54(7):739-747. doi: 10.1093/jjco/hyae037. Jpn J Clin Oncol. 2024. PMID: 38555494 Review.
Cited by
- GPCR-Hippo Signaling in Cancer.
Luo J, Yu FX. Luo J, et al. Cells. 2019 May 8;8(5):426. doi: 10.3390/cells8050426. Cells. 2019. PMID: 31072060 Free PMC article. Review. - Epithelioid hemangioendothelioma (EHE) with WWTR1::TFE3 gene fusion, a novel fusion variant.
Li S, Dermawan JK, Seavey CN, Ma S, Antonescu CR, Rubin BP. Li S, et al. Genes Chromosomes Cancer. 2024 Feb;63(2):e23226. doi: 10.1002/gcc.23226. Genes Chromosomes Cancer. 2024. PMID: 38380774 Free PMC article. - Recurrent YAP1 and MAML2 Gene Rearrangements in Retiform and Composite Hemangioendothelioma.
Antonescu CR, Dickson BC, Sung YS, Zhang L, Suurmeijer AJH, Stenzinger A, Mechtersheimer G, Fletcher CDM. Antonescu CR, et al. Am J Surg Pathol. 2020 Dec;44(12):1677-1684. doi: 10.1097/PAS.0000000000001575. Am J Surg Pathol. 2020. PMID: 32991341 Free PMC article. - Direct and selective pharmacological disruption of the YAP-TEAD interface by IAG933 inhibits Hippo-dependent and RAS-MAPK-altered cancers.
Chapeau EA, Sansregret L, Galli GG, Chène P, Wartmann M, Mourikis TP, Jaaks P, Baltschukat S, Barbosa IAM, Bauer D, Brachmann SM, Delaunay C, Estadieu C, Faris JE, Furet P, Harlfinger S, Hueber A, Jiménez Núñez E, Kodack DP, Mandon E, Martin T, Mesrouze Y, Romanet V, Scheufler C, Sellner H, Stamm C, Sterker D, Tordella L, Hofmann F, Soldermann N, Schmelzle T. Chapeau EA, et al. Nat Cancer. 2024 Jul;5(7):1102-1120. doi: 10.1038/s43018-024-00754-9. Epub 2024 Apr 2. Nat Cancer. 2024. PMID: 38565920 Free PMC article. - Unraveling the Biology of Epithelioid Hemangioendothelioma, a TAZ-CAMTA1 Fusion Driven Sarcoma.
Seavey CN, Pobbati AV, Rubin BP. Seavey CN, et al. Cancers (Basel). 2022 Jun 16;14(12):2980. doi: 10.3390/cancers14122980. Cancers (Basel). 2022. PMID: 35740643 Free PMC article. Review.
References
- Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3:98ra82. - PubMed
- Mendlick MR, Nelson M, Pickering D, Johansson SL, Seemayer TA, Neff JR, et al. Translocation t(1;3)(p36.3;q25) is a nonrandom aberration in epithelioid hemangioendothelioma. Am J Surg Pathol. 2001;25:684–687. - PubMed
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases