Kabuki syndrome genes KMT2D and KDM6A: functional analyses demonstrate critical roles in craniofacial, heart and brain development - PubMed (original) (raw)
. 2015 Aug 1;24(15):4443-53.
doi: 10.1093/hmg/ddv180. Epub 2015 May 13.
Affiliations
- PMID: 25972376
- PMCID: PMC4492403
- DOI: 10.1093/hmg/ddv180
Kabuki syndrome genes KMT2D and KDM6A: functional analyses demonstrate critical roles in craniofacial, heart and brain development
Peter M Van Laarhoven et al. Hum Mol Genet. 2015.
Abstract
Kabuki syndrome (KS) is a rare multiple congenital anomaly syndrome characterized by distinctive facial features, global developmental delay, intellectual disability and cardiovascular and musculoskeletal abnormalities. While mutations in KMT2D have been identified in a majority of KS patients, a few patients have mutations in KDM6A. We analyzed 40 individuals clinically diagnosed with KS for mutations in KMT2D and KDM6A. Mutations were detected in KMT2D in 12 and KDM6A in 4 cases, respectively. Observed mutations included single-nucleotide variations and indels leading to frame shifts, nonsense, missense or splice-site alterations. In two cases, we discovered overlapping chromosome X microdeletions containing KDM6A. To further elucidate the functional roles of KMT2D and KDM6A, we knocked down the expression of their orthologs in zebrafish. Following knockdown of kmt2d and the two zebrafish paralogs kdm6a and kdm6al, we analyzed morphants for developmental abnormalities in tissues that are affected in individuals with KS, including craniofacial structures, heart and brain. The kmt2d morphants exhibited severe abnormalities in all tissues examined. Although the kdm6a and kdm6al morphants had similar brain abnormalities, kdm6a morphants exhibited craniofacial phenotypes, whereas kdm6al morphants had prominent defects in heart development. Our results provide further support for the similar roles of KMT2D and KDM6A in the etiology of KS by using a vertebrate model organism to provide direct evidence of their roles in the development of organs and tissues affected in KS patients.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Figure 1.
Spectrum of mutations in subjects diagnosed with KS. (A) An ideogram of chromosome X is shown, highlighting a region of Xp11.3–p11.23 with a red box. An expanded view of this region is shown including the genes and chromosomal coordinates. The microdeletions detected in Subjects 1 and 2 are shown as black bars. (B) The KMT2D and KDM6A proteins are shown as gray bars. The functional domains in each protein are indicated by colored bars. The location of alterations, in both proteins, detected in Subjects 3–16 are indicated by vertical black lines.
Figure 2.
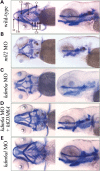
Defects in craniofacial development observed in morphant zebrafish embryos. Five day post-fertilization embryos were treated with alcian and alizarin dyes, which stain cartilage blue and bone red. Both ventral and left sagittal views focused on the visceral cranial cartilages at 10× magnification are shown. (A) Un-injected, wild-type control; (B) A typical kmt2d morphant; (C) A typical kdm6a morphant; (D) A kdm6a morphant coinjected with human KDM6A; (E) A typical kdm6al morphant. Bb, basibranchial; Cb, ceratobranchial arches 3–7; Ch, ceratohyal; O, opercle; Eth, ethmoid plate; M, Meckel's cartilage.
Figure 3.
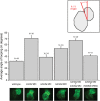
Defects in heart looping observed in morphant zebrafish embryos. Images shown of hearts from 48 h post-fertilization zebrafish embryos in transgenic line Tg(szhand2:mCherry; cmlc2:gfp)co10 which expresses green fluorescent protein (GFP) under the cmlc2 promoter were analyzed. The looping angle of the heart in the embryos was measured as shown (inset). The measured looping angles are shown as a bar graph for the wild-type and morphant embryos along with a kdm6al morphant coinjected with human KDM6A mRNA. The number of embryos (n) tested in each category are shown. Error bars represent the standard error of the mean. The bottom panel shows representative GFP-expressing hearts from each of the respective categories in the graph.
Figure 4.
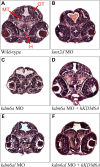
Defects in brain morphology observed in morphant zebrafish embryos. Hematoxylin and eosin (H&E) stained transverse sections of the zebrafish containing elements of both fore- and mid-brain at 48 h post-fertilization. (A) Wild-type control, (B) a typical kmt2d morphant, (C) a typical kdm6a morphant, (D) a typical kdm6a morphant coinjected with human KDM6A mRNA, (E) a typical kdm6al morphant and (F) a typical kdm6al morphant coinjected with human KDM6A mRNA_._ H, hypothalamus; MT, midbrain tegmentum; OT, optic tectum.
Figure 5.
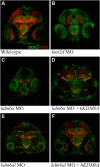
Expression of sox2 and huc in morphant zebrafish embryo brains. Transverse sections through the brain of 48 h post-fertilization zebrafish embryos immunofluorescently labeled with anti-sox2 (green) and anti-huc (red) antibodies. (A) Wild-type control, (B) a typical kmt2d morphant, (C) a typical kdm6a morphant, (D) a typical kdm6a morphant coinjected with human KDM6A mRNA, (E) a typical kdm6al morphant and (F) a typical kdm6al morphant coinjected with human KDM6A mRNA.
Similar articles
- Mutation Update for Kabuki Syndrome Genes KMT2D and KDM6A and Further Delineation of X-Linked Kabuki Syndrome Subtype 2.
Bögershausen N, Gatinois V, Riehmer V, Kayserili H, Becker J, Thoenes M, Simsek-Kiper PÖ, Barat-Houari M, Elcioglu NH, Wieczorek D, Tinschert S, Sarrabay G, Strom TM, Fabre A, Baynam G, Sanchez E, Nürnberg G, Altunoglu U, Capri Y, Isidor B, Lacombe D, Corsini C, Cormier-Daire V, Sanlaville D, Giuliano F, Le Quan Sang KH, Kayirangwa H, Nürnberg P, Meitinger T, Boduroglu K, Zoll B, Lyonnet S, Tzschach A, Verloes A, Di Donato N, Touitou I, Netzer C, Li Y, Geneviève D, Yigit G, Wollnik B. Bögershausen N, et al. Hum Mutat. 2016 Sep;37(9):847-64. doi: 10.1002/humu.23026. Epub 2016 Jul 7. Hum Mutat. 2016. PMID: 27302555 - Expanding the Oro-Dental and Mutational Spectra of Kabuki Syndrome and Expression of KMT2D and KDM6A in Human Tooth Germs.
Porntaveetus T, Abid MF, Theerapanon T, Srichomthong C, Ohazama A, Kawasaki K, Kawasaki M, Suphapeetiporn K, Sharpe PT, Shotelersuk V. Porntaveetus T, et al. Int J Biol Sci. 2018 Mar 9;14(4):381-389. doi: 10.7150/ijbs.23517. eCollection 2018. Int J Biol Sci. 2018. PMID: 29725259 Free PMC article. - Identification of KMT2D and KDM6A mutations by exome sequencing in Korean patients with Kabuki syndrome.
Cheon CK, Sohn YB, Ko JM, Lee YJ, Song JS, Moon JW, Yang BK, Ha IS, Bae EJ, Jin HS, Jeong SY. Cheon CK, et al. J Hum Genet. 2014 Jun;59(6):321-5. doi: 10.1038/jhg.2014.25. Epub 2014 Apr 17. J Hum Genet. 2014. PMID: 24739679 - Unraveling molecular pathways shared by Kabuki and Kabuki-like syndromes.
Lintas C, Persico AM. Lintas C, et al. Clin Genet. 2018 Oct;94(3-4):283-295. doi: 10.1111/cge.12983. Epub 2017 Mar 1. Clin Genet. 2018. PMID: 28139835 Review. - [One novel pathologic variation in KMT2D cause Kabuki syndrome with hearing loss as the main phenotype and related research on types of deafness].
Qiu SW, Yuan YY. Qiu SW, et al. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019 Sep;33(9):820-824. doi: 10.13201/j.issn.1001-1781.2019.09.006. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019. PMID: 31446696 Review. Chinese.
Cited by
- A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease.
Davis EJ, Broestl L, Abdulai-Saiku S, Worden K, Bonham LW, Miñones-Moyano E, Moreno AJ, Wang D, Chang K, Williams G, Garay BI, Lobach I, Devidze N, Kim D, Anderson-Bergman C, Yu GQ, White CC, Harris JA, Miller BL, Bennett DA, Arnold AP, De Jager PL, Palop JJ, Panning B, Yokoyama JS, Mucke L, Dubal DB. Davis EJ, et al. Sci Transl Med. 2020 Aug 26;12(558):eaaz5677. doi: 10.1126/scitranslmed.aaz5677. Sci Transl Med. 2020. PMID: 32848093 Free PMC article. - Role of epigenetics and miRNAs in orofacial clefts.
Garland MA, Sun B, Zhang S, Reynolds K, Ji Y, Zhou CJ. Garland MA, et al. Birth Defects Res. 2020 Nov;112(19):1635-1659. doi: 10.1002/bdr2.1802. Epub 2020 Sep 14. Birth Defects Res. 2020. PMID: 32926553 Free PMC article. Review. - Tooth agenesis and orofacial clefting: genetic brothers in arms?
Phan M, Conte F, Khandelwal KD, Ockeloen CW, Bartzela T, Kleefstra T, van Bokhoven H, Rubini M, Zhou H, Carels CE. Phan M, et al. Hum Genet. 2016 Dec;135(12):1299-1327. doi: 10.1007/s00439-016-1733-z. Epub 2016 Oct 3. Hum Genet. 2016. PMID: 27699475 Free PMC article. Review. - Small molecule inhibition of RAS/MAPK signaling ameliorates developmental pathologies of Kabuki Syndrome.
Tsai IC, McKnight K, McKinstry SU, Maynard AT, Tan PL, Golzio C, White CT, Price DJ, Davis EE, Amrine-Madsen H, Katsanis N. Tsai IC, et al. Sci Rep. 2018 Jul 17;8(1):10779. doi: 10.1038/s41598-018-28709-y. Sci Rep. 2018. PMID: 30018450 Free PMC article. - Suleiman-El-Hattab syndrome: a histone modification disorder caused by TASP1 deficiency.
Riedhammer KM, Burgemeister AL, Cantagrel V, Amiel J, Siquier-Pernet K, Boddaert N, Hertecant J, Kannouche PL, Pouvelle C, Htun S, Slavotinek AM, Beetz C, Diego-Alvarez D, Kampe K, Fleischer N, Awamleh Z, Weksberg R, Kopajtich R, Meitinger T, Suleiman J, El-Hattab AW. Riedhammer KM, et al. Hum Mol Genet. 2022 Sep 10;31(18):3083-3094. doi: 10.1093/hmg/ddac098. Hum Mol Genet. 2022. PMID: 35512351 Free PMC article.
References
- Niikawa N., Kuroki Y., Kajii T., Matsuura N., Ishikiriyama S., Tonoki H., Ishikawa N., Yamada Y., Fujita M., Umemoto H. (1988) Kabuki make-up (Niikawa-Kuroki) syndrome: a study of 62 patients. Am. J. Med. Genet., 31, 565–589. - PubMed
- Adam M., Hudgins L. (2005) Kabuki syndrome: a review. Clin. Genet., 67, 209–219. - PubMed
- Ciprero K.L., Clayton-Smith J., Donnai D., Zimmerman R.A., Zackai E.H., Ming J.E. (2005) Symptomatic Chiari I malformation in Kabuki syndrome. Am. J. Med. Genet., 132, 273–275. - PubMed
- Yano S., Matsuishi T., Yoshino M., Kato H., Kojima K. (1997) Cerebellar and brainstem ‘atrophy’ in a patient with Kabuki make-up syndrome. Am. J. Med. Genet., 71, 486–487. - PubMed
- Takano T., Matsuwake K., Yoshioka S., Takeuchi Y. (2010) Congenital polymicrogyria including the perisylvian region in early childhood. Congenit. Anom. (Kyoto), 50, 64–67. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- T32 MH015442/MH/NIMH NIH HHS/United States
- NS5048154/NS/NINDS NIH HHS/United States
- GM081519/GM/NIGMS NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous