Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles - PubMed (original) (raw)
. 2015 May 13;17(5):672-80.
doi: 10.1016/j.chom.2015.04.002.
Kristine A Kuhn 2, David L Donermeyer 2, Nathan T Porter 3, Chunsheng Jin 4, Elizabeth A Cameron 3, Haerin Jung 2, Gerard E Kaiko 2, Marta Wegorzewska 2, Nicole P Malvin 2, Robert W P Glowacki 3, Gunnar C Hansson 4, Paul M Allen 5, Eric C Martens 6, Thaddeus S Stappenbeck 7
Affiliations
- PMID: 25974305
- PMCID: PMC4432250
- DOI: 10.1016/j.chom.2015.04.002
Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles
Christina A Hickey et al. Cell Host Microbe. 2015.
Abstract
Microbes interact with the host immune system via several potential mechanisms. One essential step for each mechanism is the method by which intestinal microbes or their antigens access specific host immune cells. Using genetically susceptible mice (dnKO) that develop spontaneous, fulminant colitis, triggered by Bacteroides thetaiotaomicron (B. theta), we investigated the mechanism of intestinal microbial access under conditions that stimulate colonic inflammation. B. theta antigens localized to host immune cells through outer membrane vesicles (OMVs) that harbor bacterial sulfatase activity. We deleted the anaerobic sulfatase maturating enzyme (anSME) from B. theta, which is required for post-translational activation of all B. theta sulfatase enzymes. This bacterial mutant strain did not stimulate colitis in dnKO mice. Lastly, access of B. theta OMVs to host immune cells was sulfatase dependent. These data demonstrate that bacterial OMVs and associated enzymes promote inflammatory immune stimulation in genetically susceptible hosts.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1
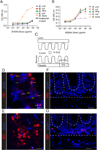
Extracellular bacterial antigen from WT B. theta localizes to the host peri-cryptal mesenchyme in dnKO mice. (A–B) Specificity of (A) 3H2 and (B) 6E9 antibody to different Bacteroides species (B. uniformis, B. vulgatus, B. TP5, B. theta) measured via ELISA at OD of 450 nm at increasing doses of antibody. Controls include (A) acapsular B. theta mutant and (A–B) E. coli. (C) Cartoon of the mouse colon indicating the location of images in (D–G). D,E= lumen, F,G=mucosa. (D–F) Sections of the colonic lumen (D, E) and mucosa (F, G) from dnKO mice 3 weeks after gavage with WT B. theta stained with 3H2 conjugated to Alexa 647 (D, F) and 6E9 conjugated to Alexa 594 antibodies, both in red (E, G). DAPI indicates nuclei in blue. Bars=3 µm (D, E). Bars=50 µm (F,G). See also Figure S1.
Figure 2
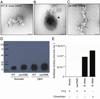
Host-penetrant B. theta antigen localizes to outer membrane vesicles (OMVs) with sulfatase activity. (A–C) Transmission electron microscopy (TEM) image of cultured (A) WT or (C) ΔanSME B. theta strains on grids without sectioning. Arrows=OMVs. (B) Cryo-sections of pelleted fecal material obtained from a dnKO mouse gavaged with WT B. theta stained with mouse 6E9 antibody followed by secondary goat anti-mouse IgG antibody conjugated to18 nm colloidal gold. Image shows a transverse cross-sectional view of B. theta parent microbe with 6E9 staining on the bacterial membrane and a budding OMV also with 6E9 staining. Arrows= OMVs, Arrowhead=B. theta. (A and C) Bars=100nm. (B) Bar=500nm. (D) Immunoblot of WT and ΔanSME B. theta sonicates and OMV preps stained with the 6E9 antibody. (E) Sulfatase activity (mol.min.mg−1) of WT and ΔanSME B. theta lysates and OMVs grown in tryptone glucose yeast (TYG) and chondroitin media. Control is TYG media alone. One representative experiment shown of n=2. See also Figure S2 and Table S1.
Figure 3
The B. theta anSME gene is necessary and sufficient for causing colitis in dnKO mice. (A) Hematoxylin and eosin (H&E) stained rectal sections from dnKO and littermate controls (IL10rb+/−) 3 weeks after gavage with B. theta strains or PBS. dnKO mice were gavaged with PBS (A1), WT B. theta (A3), ΔanSME (A4), and ΔanSME::anSME (A5), and a littermate control (IL10rb+/−) was gavaged with WT B. theta (A2). For each low-power image (100×) shown per group, a high-power image (400×) is included (boxed region adjacent to the lowpower image). Bars=200 µm for 100× images. Bars=30 µm for 400× images. (B and C) Graphs of average (B) crypts per 400× field (0.55 mm) and (C) M-phase cells per 100 crypts are shown for different groups of gavaged dnKO mice. (D) Graph of colonization at day 4 of dnKO mice and littermate control by B. theta strains via qPCR. One-way ANOVA analysis: (B) F=15.70, P<0.0001, n≥7 per group; (C) F=21.79, P<0.0001, n≥7 per group; (D) F=116.4, P<0.0001, n≥9 per group. Means with different letters are significantly different by Tukey’s multiple comparisons test. See also Figure S3.
Figure 4
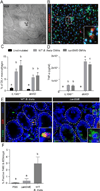
Outer membrane vesicles (OMVs) gain access to host macrophages in _B. theta_-colonized dnKO mice. (A) TEM image of 6E9 positive vesicle located within a cell consistent with a macrophage in the colon of a WT _B. theta_-gavaged dnKO mouse labeled with 6E9 mAb/goat anti-mouse IgG antibody conjugated to 18 nm colloidal gold. Bar=100nm. (B) Co-localization of macrophages (CFSE, green) derived from dnKO mice cultured with OMVs from WT B. theta (Dil Vybrant dye, red). Bar=50 µm. Bar=5 µm (inset). (C) Graph of the percent of CFSE+ macrophages that co-localized with OMVs. Unstimulated macrophages from IL10rb+/− and dnKO mice were used as a control. (D) Concentration of TNF-α (pg/ml) in the macrophage supernatant from IL10rb+/− or dnKO mice cultured with OMVs from WT or ΔanSME B. theta. (E) Staining of colonic mucosa from dnKOs gavaged with WT or ΔanSME B. theta with F4/80 (green) and 6E9 (red) antibodies. White dashed lines=outlined crypts. Bar=20 µm. Bar=2.5 µm (inset). (F) Graph of percentage of double positive F4/80+ and 6E9+ cells per crypt-associated mesenchyme in dnKO gavaged with PBS or B. theta strains. One-way ANOVA analysis: (C) F=10.65, P<0.0001, n=4 per group; (D) F=96.11, P<0.0001, n=4 per group; (F) F=5.86, P=0.01, n≥6 per group. Means with different letters are significantly different by Tukey’s multiple comparisons test. See also Figure S4.
Comment in
- Friend turned foe: a role for bacterial sulfatases in colitis.
Chatzidaki-Livanis M, Comstock LE. Chatzidaki-Livanis M, et al. Cell Host Microbe. 2015 May 13;17(5):540-1. doi: 10.1016/j.chom.2015.04.012. Cell Host Microbe. 2015. PMID: 25974293 - Mucosal immunology: Message in a bottle.
Leavy O. Leavy O. Nat Rev Immunol. 2015 Jul;15(7):402. doi: 10.1038/nri3872. Epub 2015 Jun 5. Nat Rev Immunol. 2015. PMID: 26052096 No abstract available. - Bacterial pathogenesis. Message in a bottle.
Leavy O. Leavy O. Nat Rev Microbiol. 2015 Jul;13(7):400. doi: 10.1038/nrmicro3510. Epub 2015 Jun 8. Nat Rev Microbiol. 2015. PMID: 26052665 No abstract available.
Similar articles
- Friend turned foe: a role for bacterial sulfatases in colitis.
Chatzidaki-Livanis M, Comstock LE. Chatzidaki-Livanis M, et al. Cell Host Microbe. 2015 May 13;17(5):540-1. doi: 10.1016/j.chom.2015.04.012. Cell Host Microbe. 2015. PMID: 25974293 - Bacterial pathogenesis. Message in a bottle.
Leavy O. Leavy O. Nat Rev Microbiol. 2015 Jul;13(7):400. doi: 10.1038/nrmicro3510. Epub 2015 Jun 8. Nat Rev Microbiol. 2015. PMID: 26052665 No abstract available. - Mucosal immunology: Message in a bottle.
Leavy O. Leavy O. Nat Rev Immunol. 2015 Jul;15(7):402. doi: 10.1038/nri3872. Epub 2015 Jun 5. Nat Rev Immunol. 2015. PMID: 26052096 No abstract available. - Fantastic voyage: the journey of intestinal microbiota-derived microvesicles through the body.
Stentz R, Carvalho AL, Jones EJ, Carding SR. Stentz R, et al. Biochem Soc Trans. 2018 Oct 19;46(5):1021-1027. doi: 10.1042/BST20180114. Epub 2018 Aug 28. Biochem Soc Trans. 2018. PMID: 30154095 Free PMC article. Review. - Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based.
Cai W, Kesavan DK, Wan J, Abdelaziz MH, Su Z, Xu H. Cai W, et al. Diagn Pathol. 2018 Dec 11;13(1):95. doi: 10.1186/s13000-018-0768-y. Diagn Pathol. 2018. PMID: 30537996 Free PMC article. Review.
Cited by
- Bacterial outer membrane vesicles as a candidate tumor vaccine platform.
Wang S, Guo J, Bai Y, Sun C, Wu Y, Liu Z, Liu X, Wang Y, Wang Z, Zhang Y, Hao H. Wang S, et al. Front Immunol. 2022 Sep 9;13:987419. doi: 10.3389/fimmu.2022.987419. eCollection 2022. Front Immunol. 2022. PMID: 36159867 Free PMC article. Review. - The Pros and Cons of Using Algal Polysaccharides as Prebiotics.
Gotteland M, Riveros K, Gasaly N, Carcamo C, Magne F, Liabeuf G, Beattie A, Rosenfeld S. Gotteland M, et al. Front Nutr. 2020 Sep 22;7:163. doi: 10.3389/fnut.2020.00163. eCollection 2020. Front Nutr. 2020. PMID: 33072794 Free PMC article. Review. - Development and maintenance of intestinal regulatory T cells.
Tanoue T, Atarashi K, Honda K. Tanoue T, et al. Nat Rev Immunol. 2016 May;16(5):295-309. doi: 10.1038/nri.2016.36. Epub 2016 Apr 18. Nat Rev Immunol. 2016. PMID: 27087661 Review. - Extracellular membrane vesicles in the three domains of life and beyond.
Gill S, Catchpole R, Forterre P. Gill S, et al. FEMS Microbiol Rev. 2019 May 1;43(3):273-303. doi: 10.1093/femsre/fuy042. FEMS Microbiol Rev. 2019. PMID: 30476045 Free PMC article. Review. - The NQR Complex Regulates the Immunomodulatory Function of Bacteroides thetaiotaomicron.
Engelhart MJ, Glowacki RWP, Till JM, Harding CV, Martens EC, Ahern PP. Engelhart MJ, et al. J Immunol. 2023 Sep 1;211(5):767-781. doi: 10.4049/jimmunol.2200892. J Immunol. 2023. PMID: 37486212 Free PMC article.
References
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6:498–510. - PMC - PubMed
- Berteau O, Guillot A, Benjdia A, Rabot S. A new type of bacterial sulfatase reveals a novel maturation pathway in prokaryotes. The Journal of biological chemistry. 2006;281:22464–22470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12-HD000850/HD/NICHD NIH HHS/United States
- T32 GM145304/GM/NIGMS NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- R01 GM099513/GM/NIGMS NIH HHS/United States
- DK097079/DK/NIDDK NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
- R01 DK097079/DK/NIDDK NIH HHS/United States
- T32 GM008353/GM/NIGMS NIH HHS/United States
- P30DK052574/DK/NIDDK NIH HHS/United States
- U01 AI095473/AI/NIAID NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources