Manganese Is Essential for Neuronal Health - PubMed (original) (raw)
Review
Manganese Is Essential for Neuronal Health
Kyle J Horning et al. Annu Rev Nutr. 2015.
Abstract
The understanding of manganese (Mn) biology, in particular its cellular regulation and role in neurological disease, is an area of expanding interest. Mn is an essential micronutrient that is required for the activity of a diverse set of enzymatic proteins (e.g., arginase and glutamine synthase). Although necessary for life, Mn is toxic in excess. Thus, maintaining appropriate levels of intracellular Mn is critical. Unlike other essential metals, cell-level homeostatic mechanisms of Mn have not been identified. In this review, we discuss common forms of Mn exposure, absorption, and transport via regulated uptake/exchange at the gut and blood-brain barrier and via biliary excretion. We present the current understanding of cellular uptake and efflux as well as subcellular storage and transport of Mn. In addition, we highlight the Mn-dependent and Mn-responsive pathways implicated in the growing evidence of its role in Parkinson's disease and Huntington's disease. We conclude with suggestions for future focuses of Mn health-related research.
Keywords: blood-brain barrier; cofactor; homeostasis; intracellular trafficking; metal transport; neurodevelopment.
Figures
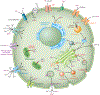
Figure 1
Known transporters and channels permeable to Mn within the brain and their cellular localizations. Other metals with affinities for each transporter are also listed. Figure is not drawn to scale. Abbreviations: DAT, dopamine transporter; ER; endoplasmic reticulum; Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; Mn, manganese; MT, metallothionein; NCX, sodium-calcium exchanger; OAT, organic anion transporter; SOCC, store-operated calcium channel.
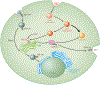
Figure 2
Mechanism of Mn detection via SPCA1 and GPP130. Endocytosed vesicles containing GPP130 are typically sorted directly to the late Golgi. SPCA1 transports Mn into the Golgi complex. In the presence of Mn and GPP130, vesicles are either guided to lysosomes, where Mn is then sequestered, or are brought to the membrane, where Mn is then exocytosed. Abbreviations: GPP, _cis_-Golgi glycoprotein; Mn, manganese; SPCA1, secretory pathway Ca2+-ATPase isoform 1.
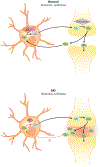
Figure 3
Glutamine synthetase activity is diminished in Huntington’s disease (HD). Glutamine synthetase is an Mn-dependent enzyme. Efficient glutamate uptake by astrocytes relies on glutamine synthetase. In HD, where cellular Mn is reduced, impaired glutamine synthetase activity inhibits glutamate uptake. Abbreviations: Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; Mn, manganese.
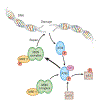
Figure 4
Mn-ATM-p53 pathway. Following doubled-stranded DNA damage, ATM is activated by Mn to phosphorylate p53 and Mre11 of the MRN complex. The MRN complex is then able to repair the DNA damage. Abbreviations: ATM, ataxia telangiectasia mutated; Mn, manganese; MRN, Mre11, Rad50, Nbs1.
Figure 5
Inflammatory response induced by Mn. Mn exposure leads to the production of NF-κB and p38 in astrocytes, which increases reactive oxygen species. Inflammatory markers (IL-6, IL-1β, and TNF-α) are produced by microglia following Mn exposure. In the presence of these markers and Mn, neurons have been found to increase expression of the Mn-permeable ZIP14 and decrease expression of the Mn exporter SLC30A10. Abbreviations: IL-1β/6, interleukin-1β/6; Mn, manganese; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; ROS, reactive oxygen species; SLC, solute carrier; TNFα; tumor necrosis factor-alpha.
Similar articles
- Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington's disease mouse model.
Bichell TJV, Wegrzynowicz M, Tipps KG, Bradley EM, Uhouse MA, Bryan M, Horning K, Fisher N, Dudek K, Halbesma T, Umashanker P, Stubbs AD, Holt HK, Kwakye GF, Tidball AM, Colbran RJ, Aschner M, Neely MD, Di Pardo A, Maglione V, Osmand A, Bowman AB. Bichell TJV, et al. Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1596-1604. doi: 10.1016/j.bbadis.2017.02.013. Epub 2017 Feb 16. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28213125 Free PMC article. - Brain manganese and the balance between essential roles and neurotoxicity.
Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M, Haynes EN, Bowman AB. Balachandran RC, et al. J Biol Chem. 2020 May 8;295(19):6312-6329. doi: 10.1074/jbc.REV119.009453. Epub 2020 Mar 18. J Biol Chem. 2020. PMID: 32188696 Free PMC article. Review. - Huntington's disease associated resistance to Mn neurotoxicity is neurodevelopmental stage and neuronal lineage dependent.
Joshi P, Bodnya C, Ilieva I, Neely MD, Aschner M, Bowman AB. Joshi P, et al. Neurotoxicology. 2019 Dec;75:148-157. doi: 10.1016/j.neuro.2019.09.007. Epub 2019 Sep 20. Neurotoxicology. 2019. PMID: 31545971 Free PMC article. - Altered manganese homeostasis and manganese toxicity in a Huntington's disease striatal cell model are not explained by defects in the iron transport system.
Williams BB, Kwakye GF, Wegrzynowicz M, Li D, Aschner M, Erikson KM, Bowman AB. Williams BB, et al. Toxicol Sci. 2010 Sep;117(1):169-79. doi: 10.1093/toxsci/kfq174. Epub 2010 Jun 13. Toxicol Sci. 2010. PMID: 20547568 Free PMC article. - Manganese homeostasis in the nervous system.
Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M. Chen P, et al. J Neurochem. 2015 Aug;134(4):601-10. doi: 10.1111/jnc.13170. Epub 2015 Jun 16. J Neurochem. 2015. PMID: 25982296 Free PMC article. Review.
Cited by
- Neuronal SLC39A8 deficiency impairs cerebellar development by altering manganese homeostasis.
Choi EK, Aring L, Peng Y, Correia AB, Lieberman AP, Iwase S, Seo YA. Choi EK, et al. JCI Insight. 2024 Oct 22;9(20):e168440. doi: 10.1172/jci.insight.168440. JCI Insight. 2024. PMID: 39435657 Free PMC article. - Epitranscriptomic reader YTHDF2 regulates SEK1(MAP2K4)-JNK-cJUN inflammatory signaling in astrocytes during neurotoxic stress.
Malovic E, Ealy A, Miller C, Jang A, Hsu PJ, Sarkar S, Rokad D, Goeser C, Hartman AK, Zhu A, Palanisamy B, Zenitsky G, Jin H, Anantharam V, Kanthasamy A, He C, Kanthasamy AG. Malovic E, et al. iScience. 2024 Jul 31;27(9):110619. doi: 10.1016/j.isci.2024.110619. eCollection 2024 Sep 20. iScience. 2024. PMID: 39252959 Free PMC article. - Association between heavy metals exposure and risk of attention deficit hyperactivity disorder (ADHD) in children: a systematic review and meta-analysis.
Gu Q, Liu J, Zhang X, Huang A, Yu X, Wu K, Huang Y. Gu Q, et al. Eur Child Adolesc Psychiatry. 2024 Aug 10. doi: 10.1007/s00787-024-02546-z. Online ahead of print. Eur Child Adolesc Psychiatry. 2024. PMID: 39126497 Review. - Associations of Dietary Copper, Selenium, and Manganese Intake With Depression: A Meta-Analysis of Observational Studies.
Ding J, Zhang Y. Ding J, et al. Front Nutr. 2022 Mar 15;9:854774. doi: 10.3389/fnut.2022.854774. eCollection 2022. Front Nutr. 2022. PMID: 35369103 Free PMC article. - Alterations in metal homeostasis occur prior to canonical markers in Huntington disease.
Pfalzer AC, Yan Y, Kang H, Totten M, Silverman J, Bowman AB, Erikson K, Claassen DO. Pfalzer AC, et al. Sci Rep. 2022 Jun 20;12(1):10373. doi: 10.1038/s41598-022-14169-y. Sci Rep. 2022. PMID: 35725749 Free PMC article.
References
- Aggarwal A, Vaidya S, Shah S, Singh J, Desai S, Bhatt M. 2006. Reversible Parkinsonism and T1W pallidal hyperintensities in acute liver failure. Mov. Disord 21:1986–90 - PubMed
- Akatsu H, Hori A, Yamamoto T, Yoshida M, Mimuro M, et al. 2012. Transition metal abnormalities in progressive dementias. Biometals 25:337–50 - PubMed
- Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. 1997. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. J. Parenter. Enteral Nutr 21:41–45 - PubMed
- Ambrose M, Goldstine JV, Gatti RA. 2007. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum. Mol. Genet 16:2154–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES020852/ES/NIEHS NIH HHS/United States
- T32 ES007028/ES/NIEHS NIH HHS/United States
- R01 ES16931/ES/NIEHS NIH HHS/United States
- R01 ES016931/ES/NIEHS NIH HHS/United States
- R01 ES007331/ES/NIEHS NIH HHS/United States
- R01 ES10563/ES/NIEHS NIH HHS/United States
- R01 ES010563/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources