Fever and the thermal regulation of immunity: the immune system feels the heat - PubMed (original) (raw)
Review
Fever and the thermal regulation of immunity: the immune system feels the heat
Sharon S Evans et al. Nat Rev Immunol. 2015 Jun.
Abstract
Fever is a cardinal response to infection that has been conserved in warm-blooded and cold-blooded vertebrates for more than 600 million years of evolution. The fever response is executed by integrated physiological and neuronal circuitry and confers a survival benefit during infection. In this Review, we discuss our current understanding of how the inflammatory cues delivered by the thermal element of fever stimulate innate and adaptive immune responses. We further highlight the unexpected multiplicity of roles of the pyrogenic cytokine interleukin-6 (IL-6), both during fever induction and during the mobilization of lymphocytes to the lymphoid organs that are the staging ground for immune defence. We also discuss the emerging evidence suggesting that the adrenergic signalling pathways associated with thermogenesis shape immune cell function.
Conflict of interest statement
Conflict of interest statement: The authors have no conflicting financial interests.
Figures
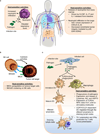
Figure 1. The induction of fever during infection
The recognition of damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), by Toll-like receptors (TLRs) and other pattern recognition receptors drives the activation of dendritic cells (DCs) and macrophages. These innate immune cells release prostaglandin E2 (PGE2) as well as pyrogenic cytokines (namely, interleukin-1 (IL-1) IL-6, and tumour necrosis factor (TNF)) that act systemically to induce fever. IL-6 operates downstream of IL-1 in the median preoptic nucleus region within the hypothalamus to induce the synthesis of cyclooxygenase 2 (COX2), the enzyme responsible for production of additional PGE2., PGE2 is considered the major pyrogenic mediator of fever.– Receptor activator of NF-κB (RANK) expressed by astrocytes also acts via the COX2–PGE2 pathway to induce fever. However, it is not known whether this pathway parallels the IL-6 response or if the IL-6 and RANKL pathways converge, potentially via IL-6 regulation of RANKL expression in vascular endothelial cells in the hypothalamus. Neurons expressing PGE2 receptor 3 (EP3) trigger the sympathetic nervous system to trigger norepinephrine release, which elevates body temperature by increasing thermogenesis in brown adipose tissue as well as by inducing vasoconstriction to prevent passive heat loss.,,,, Additionally, acetylcholine contributes to fever by stimulating muscle myocytes to induce shivering.
Figure 2. Response of innate immune cells to thermal stress
(a) Fever-range temperatures drive several crucial aspects of innate immunity. Fever-range hyperthermia stimulates the release of neutrophils from the bone marrow in a granulocyte–colony-stimulating factor (G-CSF)-driven manner.– Febrile-range temperatures also promote neutrophil recruitment to the lungs and other local sites of infection in a CXC-chemokine ligand 8 (CXCL8)-dependent fashion that additionally involves decreased barrier function of vessels.,, Upon arriving in the site of infection, thermal stress further elevates the respiratory burst which increases the bacteriolytic activity of neutrophils., (b) Thermal treatment improves natural killer (NK) cell cytolytic activity through induction of MHC class I polypeptide-related sequence A (MICA) expression on target cells (for example, tumour cells) as well as by inducing the clustering of the MICA counter-receptor NKG2D on the surface of NK cells. (c) Temperatures in the febrile range increase the ability of antigen-presenting cells to support the formation of the adaptive immune response. Heat improves the phagocytic potential of macrophages and dendritic cells (DCs) and increases their responsiveness to invading pathogens by upregulating their expression of both Toll-like receptor 2 (TLR2) and TLR4., Thermal treatment also induces the release of immunomodulatory molecules such as cytokines (for example, TNF), nitric oxide (NO) and heat shock protein 70 (HSP70). Additionally, heat increases expression of MHC class I and II molecules as well as co-stimulatory molecules (CD80 and CD86) by mature DCs and augments their CC-chemokine receptor 7 (CCR7)-dependent migration via the afferent lymphatics that serve as a conduit to draining lymph nodes.,– DCs exposed to febrile temperatures are also more efficient at cross-presenting antigens and inducing T helper 1 (Th1) cell polarization.
Figure 3. Fever-range thermal stress and the adaptive immune response
(a) Fever-range thermal stress supports increased adaptive immunity by targeting two distinct aspects of T cell activation in lymph nodes. Heat enhances the rate of lymphocyte trafficking across high endothelial venules (HEVs) in peripheral lymph nodes through effects on each step of the adhesion cascade. Heat treatment of lymphocytes increases the frequency of L-selectin-dependent tethering and rolling interactions.,,– Febrile-range temperatures independently act on HEVs to enhance the transition of lymphocytes from transient rolling to stable arrest by increasing the intravascular density of CC-chemokine ligand 21 (CCL21) and intracellular adhesion molecule 1 (ICAM1).– ICAM1 also supports lymphocyte crawling to inter-endothelial cell junctions as well as transendothelial migration.,, Heat also acts directly on the T cells within lymphoid organs by pre-clustering components of the immunological synapse (TCRβ and CD8) into lipid rafts. This prolongs stable contacts with APCs and increases CD8+ T cell differentiation towards an effector phenotype characterized by enhanced L-selectin downregulation, cytotoxic function, and production of interferon-γ (IFNγ)., (b) Epifluorescence whole-mount confocal microscopy imaging of HEVs that are actively supporting lymphocyte trafficking in a mouse lymph node. HEVs are stained in red with PE-conjugated MECA-79 antibody that recognizes peripheral lymph node addressin (PNAD) whereas lymphocytes are labelled in green using carboxyfluorescein succinimidyl ester (CFSE).
Figure 4. Thermal stress acts through IL-6 trans-signalling to improve lymphocyte trafficking into lymph nodes
(a) Heat-dependent interelukin-6 (IL-6) trans-signalling is initiated by binding of the soluble form of the IL-6 receptor α subunit (sIL-6Rα) to both IL-6 and membrane-anchored gp130., Soluble gp130 functions as a selective antagonist of IL-6 trans-signalling and downstream activation of canonical JAK–STAT and MEK1–ERK1/ERK2 signalling pathways but does not interfere with classical signalling by membrane-anchored IL-6Rα and transmembrane gp130. (b) Febrile temperatures act on lymphocytes and high endothelial cells (HECs) to improve lymphocyte trafficking exclusively across high endothelial venules (HEVs) in lymph nodes. Vessel segments immediately proximal to HEVs are refractory to thermal treatment, which may reflect the lower expression of gp130 by non-specialized squamous endothelial cells that line non-HEVs.Left inset, fever-range temperatures act directly on lymphocytes through IL-6 trans-signalling to stimulate the MEK1–ERK1/ERK2 signalling pathway, promoting L-selectin adhesion as well as intermolecular interactions between the actin-based cytoskeleton, α-actinin, and the cytoplasmic tail of L-selectin.Right inset, IL-6 trans-signalling upregulates the intravascular density of ICAM1 in HEVs during heat treatment of mice, although the downstream signalling mediators remain unknown. Fibroblastic reticular cells that are in direct contact with HECs are a possible source of the IL-6 while proximal dendritic cells (DCs) and T cells could provide the sIL-6R, required to enhance the adhesive properties of HEVs during thermal stress.
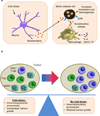
Figure 5. Cold stress stimulates nerve-driven modulation of thermogenesis and anti-tumour immunity
(a) Exposure to cold stress drives the release of neurotransmitters, such as norepinephrine, by neurons. This initiates the interleukin-4 (IL-4) and IL-13-driven ‘alternative activation’ programme of differentiation in macrophages, resulting in additional production of norepinephrine, which stimulates β-adrenergic receptors (βAR) expressed on brown adipose cells driving thermogenesis. (b) Cold stress in tumour-bearing mice maintained at standard housing temperatures (20–26°C) tilts the balance towards an immunosuppressive local tumour microenvironment. This is characterized by a substantial increase in populations of intratumoral myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells and a concomitant decrease in the number of CD8+ T cells when compared to tumours that develop in mice housed under thermoneutral ambient temperature (30–31°C). Tumour cell survival and tumour growth are also accelerated by cold stress. –
Similar articles
- The biology of innate lymphoid cells.
Artis D, Spits H. Artis D, et al. Nature. 2015 Jan 15;517(7534):293-301. doi: 10.1038/nature14189. Nature. 2015. PMID: 25592534 Review. - Fine-tuning immune surveillance by fever-range thermal stress.
Fisher DT, Vardam TD, Muhitch JB, Evans SS. Fisher DT, et al. Immunol Res. 2010 Mar;46(1-3):177-88. doi: 10.1007/s12026-009-8122-9. Immunol Res. 2010. PMID: 19760057 Free PMC article. Review. - [Mechanism for the induction of host immune response by virulence factors of Listeria monocytogenes].
Mitsuyama M. Mitsuyama M. Nihon Saikingaku Zasshi. 2009 Dec;64(2-4):365-76. doi: 10.3412/jsb.64.365. Nihon Saikingaku Zasshi. 2009. PMID: 20023369 Review. Japanese. No abstract available. - Perspective on fever: the basic science and conventional medicine.
Cannon JG. Cannon JG. Complement Ther Med. 2013 Apr;21 Suppl 1:S54-60. doi: 10.1016/j.ctim.2011.08.002. Epub 2011 Sep 17. Complement Ther Med. 2013. PMID: 23578918 Review. - Neuroimmunoregulation and natural immunity.
Berczi I, Chow DA, Sabbadini ER. Berczi I, et al. Domest Anim Endocrinol. 1998 Sep;15(5):273-81. doi: 10.1016/s0739-7240(98)00015-0. Domest Anim Endocrinol. 1998. PMID: 9785030 Review.
Cited by
- Shaping the immune landscape: Multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration.
Xue JD, Gao J, Tang AF, Feng C. Xue JD, et al. Heliyon. 2024 Aug 30;10(17):e37192. doi: 10.1016/j.heliyon.2024.e37192. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296009 Free PMC article. Review. - Modulating the Heat Stress Response to Improve Hyperthermia-Based Anticancer Treatments.
Scutigliani EM, Liang Y, Crezee H, Kanaar R, Krawczyk PM. Scutigliani EM, et al. Cancers (Basel). 2021 Mar 12;13(6):1243. doi: 10.3390/cancers13061243. Cancers (Basel). 2021. PMID: 33808973 Free PMC article. Review. - Safety of Adjuvanted Recombinant Herpes Zoster Virus Vaccination in Fragile Populations: An Observational Real-Life Study.
Costantino M, Giudice V, Moccia G, Longanella W, Caruccio S, Tremiterra G, Sinopoli P, Benvenuto D, Serio B, Malatesta F, Pecoraro N, Vozzella EA, Rossiello R, Genovese G, De Caro F. Costantino M, et al. Vaccines (Basel). 2024 Aug 29;12(9):990. doi: 10.3390/vaccines12090990. Vaccines (Basel). 2024. PMID: 39340022 Free PMC article. - Nanotechnology synergized immunoengineering for cancer.
Chauhan DS, Dhasmana A, Laskar P, Prasad R, Jain NK, Srivastava R, Jaggi M, Chauhan SC, Yallapu MM. Chauhan DS, et al. Eur J Pharm Biopharm. 2021 Jun;163:72-101. doi: 10.1016/j.ejpb.2021.03.010. Epub 2021 Mar 24. Eur J Pharm Biopharm. 2021. PMID: 33774162 Free PMC article. Review.
References
- Celsus ACA. In: Cornelius Celsus of medicine In eight books. Translated, with notes critical and explanatory, by. Greive James., MD, editor. London: printed for D. Wilson and T. Durham; 1756.
- Kluger MJ. Phylogeny of fever. Fed Proc. 1979;38:30–34. - PubMed
- Schulman CI, et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt) 2005;6:369–375. References and 4 show that treatment of fever using anti-pyretic drugs has a detrimental effect on patient outcome during infection.
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI082039/AI/NIAID NIH HHS/United States
- R01 CA079765/CA/NCI NIH HHS/United States
- CA79765/CA/NCI NIH HHS/United States
- R01 AI082039/AI/NIAID NIH HHS/United States
- CA085183/CA/NCI NIH HHS/United States
- AI082039/AI/NIAID NIH HHS/United States
- T32 CA085183/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical