Catastrophic chromosomal restructuring during genome elimination in plants - PubMed (original) (raw)
Catastrophic chromosomal restructuring during genome elimination in plants
Ek Han Tan et al. Elife. 2015.
Abstract
Genome instability is associated with mitotic errors and cancer. This phenomenon can lead to deleterious rearrangements, but also genetic novelty, and many questions regarding its genesis, fate and evolutionary role remain unanswered. Here, we describe extreme chromosomal restructuring during genome elimination, a process resulting from hybridization of Arabidopsis plants expressing different centromere histones H3. Shattered chromosomes are formed from the genome of the haploid inducer, consistent with genomic catastrophes affecting a single, laggard chromosome compartmentalized within a micronucleus. Analysis of breakpoint junctions implicates breaks followed by repair through non-homologous end joining (NHEJ) or stalled fork repair. Furthermore, mutation of required NHEJ factor DNA Ligase 4 results in enhanced haploid recovery. Lastly, heritability and stability of a rearranged chromosome suggest a potential for enduring genomic novelty. These findings provide a tractable, natural system towards investigating the causes and mechanisms of complex genomic rearrangements similar to those associated with several human disorders.
Keywords: arabidopsis; chromosome segregation; chromosomes; genes; genome instability; mitosis.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
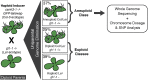
Figure 1.. Ploidy types resulting from centromere-mediated uniparental genome elimination.
The altered CENH3 ‘_GFP_-tailswap’ strain was hybridized to the recessive glabrous1-1 mutant. Mean percentages of haploid, diploid and aneuploid progeny obtained from crosses to three independent _GFP_-tailswap lines are indicated, as determined after phenotypic characterization. Individuals belonging to the aneuploid class were sequenced and subjected to chromosome dosage and single nucleotide polymorphism (SNP) analysis as indicated by the arrow. DOI:
http://dx.doi.org/10.7554/eLife.06516.003
Figure 1—figure supplement 1.. Dosage plots and SNP analysis of diploids from _GFP_-tailswap haploid induction crosses.
(A) Dosage plots from all five Arabidopsis chromosomes in consecutive non-overlapping 100 kbp bins. (B) The corresponding SNP plot for the % haploid inducer genome (Col-0) present in each sample. DOI:
http://dx.doi.org/10.7554/eLife.06516.004
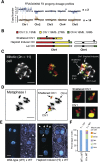
Figure 2.. Characterization of the three distinct aneuploid types from _GFP_-tailswap haploid induction crosses.
(A–D) Dosage plots from all five Arabidopsis chromosomes in consecutive non-overlapping 100 kbp bins and the corresponding SNP plot for the % haploid inducer genome (Col-0) present in each sample. A diploid Col/L_er_ hybrid (A), an individual with primary Chr3 trisomy from the numerical aneuploid class (B), an individual with a truncated trisomic Chr3 (C) and an individual with shattered Chr3 (D) are shown here. Centromere positions are indicated by red diamonds. (E) Percentages of the different aneuploid types obtained from three different _GFP_-tailswap haploid inducer lines. (F) For each chromosome, the percentage of aneuploid individuals exhibiting altered dosage for that particular chromosome is plotted. All aneuploids characterized in this study are included. Chr4 is overrepresented (**Student's _t_-test, p < 0.01) while Chr5 is underrepresented (* Student's _t_-test, p < 0.05). DOI:
http://dx.doi.org/10.7554/eLife.06516.005
Figure 2—figure supplement 1.. Copy number and T-DNA positions of the _GFP_-tailswap transgene in three independently derived transgenic lines.
(A) The three lines used in this study and the insertion sites for each _GFP_-tailswap transgene are shown. Lines #11 and #17 carry four copies of the transgene and the transformation event resulted in a reciprocal translocation of Chr1 and Chr4 for #11 and Chr1 and Chr2 for #17. Line #22 carries a single copy insertion on Chr5. (B, C) Exact positions of the insertion sites for line #11 (B) and line #17 (C). T-DNA left borders are denoted by ‘LB’. DOI:
http://dx.doi.org/10.7554/eLife.06516.006
Figure 2—figure supplement 2.. Dosage plots and SNP analysis of atypical aneuploids from _GFP_-tailswap haploid induction crosses.
(A–D). Dosage plots from all five Arabidopsis chromosomes in consecutive non-overlapping 100 kbp bins and the corresponding SNP plot for the % haploid inducer genome (Col-0) present in each sample. A monosomic Chr2 and Chr3 individual with 2n = 10 – 1 chromosomes (A, B), a disomic Chr4 individual with 2n = 5 + 1 chromosomes (C) and a haploid individual with a Chr1 minichromosome (truncated class) derived from the haploid inducer genome (D) are shown. The unusually high dosage spike around the centromere of Chr2 in FRA00870 (A) results from monosomy for Chr2. L_er_ sequences are mapped to the Col-0 genomic reference followed by normalization to our diploid control F1 hybrid Col/L_er_. Euploid chromosome dosage plots for haploids with disomic chromosomes or minichromosomes (C, D) have the appearance of having a copy number of 2 only because euploid chromosome dosage was calculated with the value of 2 in this analysis. Centromere positions are indicated by red diamonds. DOI:
http://dx.doi.org/10.7554/eLife.06516.007
Figure 2—figure supplement 3.. Diversity of primary trisomic aneuploids derived from a selfed triploid (Col-0 ecotype).
(A) Primary trisomics (2n = 10 + 1) for all five Arabidopsis chromosomes. (B–D) Higher order primary trisomics from our analysis. We observed (B) double primary trisomics (2n = 10 + 1 + 1), (C) triple primary trisomics (2n = 10 + 1 + 1 + 1) as well as (D) quadruple primary trisomics (2n = 10 + 1 + 1 + 1 + 1). ‘N/A’ indicates the karyotypes that were not observed in this population. DOI:
http://dx.doi.org/10.7554/eLife.06516.008
Figure 2—figure supplement 4.. Representative dosage plots from 96 individuals from a selfed _GFP_-tailswap haploid inducer.
Representative dosage plot of diploid individuals (94/96 = 98%) showing a euploid dosage from all five Arabidopsis chromosomes. We detected two selfed individuals with numerical whole chromosome primary trisomies (2n + 1) for Chr2 and Chr3, which are shown here. DOI:
http://dx.doi.org/10.7554/eLife.06516.009
Figure 3.. Shattered chromosomes are confined to a single chromosome originating from the haploid inducer.
(A) Chromosome dosage plots based on non-overlapping 25 kbp bins across each chromosome for five aneuploid individuals with shattered chromosomes. The _GFP_-tailswap transgene insertion event that resulted in a reciprocal translocation between Chr1 and Chr4 in one of the haploid inducer parent (_GFP_-tailswap #11) is indicated with black arrowheads. The translocation is only visible in individuals for which chromosomes 1 and 4 are not balanced with each other. Duplications (copy number 3), triplications (copy number 4) as well as deletions accompanied with loss of heterozygosity (copy number 1) were observed from dosage plots. (B) Box plots of the percentage of haploid inducer genome present at each copy number state, as determined by the SNP analysis. Mean and standard errors are shown. DOI:
http://dx.doi.org/10.7554/eLife.06516.010
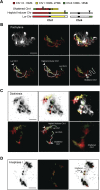
Figure 4.. Stable inheritance and chromosome painting of a shattered aneuploid chromosome.
(A) Dosage analysis from 16 F2 individuals from a selfed FRAG00062 individual. Progeny individuals either inherited the shattered chromosome intact (n = 2) or appeared diploid (n = 14). (B) Cartoon of the different versions of chromosomes 1 and 4 expected to be present in FRAG00062. Chromosome painting probes and corresponding chromosome positions used for (C) and (D) are shown. Black triangles indicate the position of the reciprocal Chr1/Chr4 translocation present in the haploid inducer line, whereas black circles indicate centromere positions. (C) A mitotic cell from FRAG00062 with 11 chromosomes, including four painted chromosomes. Scale bar = 5 μm. (D) The shattered Chr1 from FRAG00062 remains unpaired at meiosis as shown here at Metaphase I. Enlargements of the shattered Chr1 and paired Chr1 are shown on the right. Scale bar = 5 μm. (E) Nuclei from a two-cell stage embryo from a wild-type cross (left panel) and from an embryo undergoing uniparental genome elimination (right panel). Nuclei are visualized using CFP-tagged histone H2B from the pollen parent superimposed with an image of the embryo visualized under light microscopy. Note the presence of micronuclei from the embryo undergoing genome elimination (right panel). Scale bar = 5 μm. (F) Percentage of micronuclei observed in wild-type crosses and genome elimination crosses. The different percentages of micronuclei per cell are indicated. DOI:
http://dx.doi.org/10.7554/eLife.06516.011
Figure 4—figure supplement 1.. Analysis of duplicated and triplicated blocks from FRAG00062.
(A) Cartoon of duplicated and triplicated blocks on the shattered aneuploid chromosome from FRAG00062. (B) Rearranged pattern from DNA fluorescence in situ hybridization (FISH) with the corresponding de novo assembled contigs. DOI:
http://dx.doi.org/10.7554/eLife.06516.012
Figure 4—figure supplement 2.. DNA FISH on the shattered aneuploid chromosome from FRAG00062.
(A) Cartoon of the regions used for chromosome painting experiments. (B–D) Meiotic spreads from male meiocytes with DNA painting probes shown in (A). Scale bars = 5 μm. The unpaired shattered chromosome is visible in Pachytene (B) and Diakinesis (C) and separate precociously at Anaphase I (D). DOI:
http://dx.doi.org/10.7554/eLife.06516.013
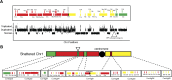
Figure 5.. Breakpoint junctions and enriched features surrounding breakpoints of duplicated and triplicated blocks.
(A) Plot of all chromosomes and corresponding dosage plots (blue line) from FRAG00062. Black curves depict novel junctions identified from genomic reconstruction of the shattered haploid inducer Chr1. Black triangles indicate the reciprocal translocation between Chr1 and Chr4 from the haploid inducer genome. (B) Percentage of junctions with 2–15 bp of microhomology, blunt junctions or junctions with unidentified sequence insertion observed from FRAG00062. (C) Plot of duplicated (n = 23) and triplicated (n = 23) block sizes from FRAG00062. (*** Student's _t_-test p < 0.001). (D, E) Occupancy of genes and replication origins around breakpoints regions, calculated using windows of 1000 bp or 10,000 bp centered on each breakpoint. Dashed horizontal lines indicate the genome-wide occupancy of each feature. Error bars indicate standard error. (F, G) Enrichment ratio of genes and replication origins (See methods for description of enrichment ratio). Genes (F) are significantly enriched surrounding duplicated breakpoints regardless of window size (1000 or 10,000 bp, **p < 0.01). For windows of 10,000 bp, replication origins (G) are significantly enriched at triplicated breakpoints (*p < 0.05) while significantly under-represented (‡p < 0.05) at duplicated breakpoints. DOI:
http://dx.doi.org/10.7554/eLife.06516.014
Figure 5—figure supplement 1.. PCR confirmation of breakpoint junctions for FRAG00062.
DNA gel showing PCR assays performed using oligo pairs from a control region and from breakpoint junctions specific to FRAG00062 using DNA from a diploid sibling (top panel) or FRAG00062 (bottom panel). DOI:
http://dx.doi.org/10.7554/eLife.06516.015
Figure 5—figure supplement 2.. Breakpoint junctions types from FRAG00062.
(A, B). Top: Junction sequence from direct sequencing of breakpoint junctions. Middle and bottom: Reference A. thaliana sequence. Sequences in common with the junction sequence are in blue and red. (+) denote sequences that align to the forward strand of the reference genome while (−) denote sequences that align to the reverse strand. Regions of microhomology are highlighted in yellow (A). Inserted sequence is highlighted in green (B). (C) Distribution of the sizes of microhomology tracts from breakpoints junctions. (D) Distribution of the sizes of unidentified inserted DNA sequences from breakpoint junctions grouped in 25 bp bins. DOI:
http://dx.doi.org/10.7554/eLife.06516.016
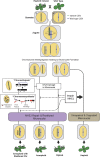
Figure 6.. The process of genome elimination and connected models for chromosomal rearrangements.
Genome elimination ensues when a haploid inducer expressing a variant CENH3 protein mates with the wild type. In many cases, the chromosomes marked by the variant CENH3 missegregate in the embryo and are compartmentalized in micronuclei. DNA damage, NHEJ repair and restitution of the micronucleus to the euploid pole nucleus can result in aneuploidy or diploidy. Alternatively, shattered chromosomes result from chromothripsis and chromoanasynthesis. The former involves fragmentation and random ligation, the latter replication fork collapse and microhomology-mediated strand switching. As a consequence, the pulverized and reassembled chromosome forms a single unit and can be meiotically inherited. The schematics for chromothripsis and chromoanasynthesis are shown sequentially for convenience, but their order has not been determined. In addition, our results obtained using DNA ligase4-2 mutants suggest that the NHEJ pathway plays an important role in the repair of the haploid inducer chromosomes that contribute to diploid and aneuploid progeny, such that when NHEJ is inhibited, haploid induction frequency increased. DOI:
http://dx.doi.org/10.7554/eLife.06516.017
Figure 7.. Dosage plots for lig4-2 haploids isolated from a haploid induction cross using diploid lig4-2 as the male donor.
Dosage plots of lig4-2 haploids based on 150 kbp non-overlapping bins across all five Arabidopsis chromosomes. Euploid chromosome dosage plots for lig4-2 haploids have the appearance of having a copy number of 2 only because euploid chromosome dosage was calculated with the value of 2 in this analysis. Centromere positions are indicated by red diamonds. DOI:
http://dx.doi.org/10.7554/eLife.06516.019
Similar articles
- Marked contribution of alternative end-joining to chromosome-translocation-formation by stochastically induced DNA double-strand-breaks in G2-phase human cells.
Soni A, Siemann M, Pantelias GE, Iliakis G. Soni A, et al. Mutat Res Genet Toxicol Environ Mutagen. 2015 Nov;793:2-8. doi: 10.1016/j.mrgentox.2015.07.002. Epub 2015 Jul 4. Mutat Res Genet Toxicol Environ Mutagen. 2015. PMID: 26520366 - Detection of Chromothripsis in Plants.
Henry IM, Comai L, Tan EH. Henry IM, et al. Methods Mol Biol. 2018;1769:119-132. doi: 10.1007/978-1-4939-7780-2_8. Methods Mol Biol. 2018. PMID: 29564821 - The Arabidopsis AtLIG4 gene is required for the repair of DNA damage, but not for the integration of Agrobacterium T-DNA.
van Attikum H, Bundock P, Overmeer RM, Lee LY, Gelvin SB, Hooykaas PJ. van Attikum H, et al. Nucleic Acids Res. 2003 Jul 15;31(14):4247-55. doi: 10.1093/nar/gkg458. Nucleic Acids Res. 2003. PMID: 12853643 Free PMC article. - Mechanisms of DNA double strand break repair and chromosome aberration formation.
Iliakis G, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, Wu W, Guan J, Terzoudi G, Pantelias G. Iliakis G, et al. Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review. - [Double strand break repair, one mechanism can hide another: alternative non-homologous end joining].
Rass E, Grabarz A, Bertrand P, Lopez BS. Rass E, et al. Cancer Radiother. 2012 Feb;16(1):1-10. doi: 10.1016/j.canrad.2011.05.004. Epub 2011 Jul 6. Cancer Radiother. 2012. PMID: 21737335 Review. French.
Cited by
- Synthetic apomixis enables stable transgenerational transmission of heterotic phenotypes in hybrid rice.
Liu C, He Z, Zhang Y, Hu F, Li M, Liu Q, Huang Y, Wang J, Zhang W, Wang C, Wang K. Liu C, et al. Plant Commun. 2023 Mar 13;4(2):100470. doi: 10.1016/j.xplc.2022.100470. Epub 2022 Nov 2. Plant Commun. 2023. PMID: 36325606 Free PMC article. - Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing.
Cortés-Ciriano I, Lee JJ, Xi R, Jain D, Jung YL, Yang L, Gordenin D, Klimczak LJ, Zhang CZ, Pellman DS; PCAWG Structural Variation Working Group; Park PJ; PCAWG Consortium. Cortés-Ciriano I, et al. Nat Genet. 2020 Mar;52(3):331-341. doi: 10.1038/s41588-019-0576-7. Epub 2020 Feb 5. Nat Genet. 2020. PMID: 32025003 Free PMC article. - Wheat Cybrid Plants, OryzaWheat, Regenerated from Wheat-Rice Hybrid Zygotes via in Vitro Fertilization System Possess Wheat-Rice Hybrid Mitochondria.
Maryenti T, Koshimizu S, Onda N, Ishii T, Yano K, Okamoto T. Maryenti T, et al. Plant Cell Physiol. 2024 Sep 3;65(8):1344-1357. doi: 10.1093/pcp/pcae074. Plant Cell Physiol. 2024. PMID: 39107984 Free PMC article. - Twenty-Five Years of Propagation in Suspension Cell Culture Results in Substantial Alterations of the Arabidopsis Thaliana Genome.
Pucker B, Rückert C, Stracke R, Viehöver P, Kalinowski J, Weisshaar B. Pucker B, et al. Genes (Basel). 2019 Sep 2;10(9):671. doi: 10.3390/genes10090671. Genes (Basel). 2019. PMID: 31480756 Free PMC article. - Effect of Hybridization on Somatic Mutations and Genomic Rearrangements in Plants.
Bashir T, Chandra Mishra R, Hasan MM, Mohanta TK, Bae H. Bashir T, et al. Int J Mol Sci. 2018 Nov 27;19(12):3758. doi: 10.3390/ijms19123758. Int J Mol Sci. 2018. PMID: 30486351 Free PMC article. Review.
References
- Costas C, de la Paz Sanchez M, Stroud H, Yu Y, Oliveros JC, Feng S, Benguria A, López-Vidriero I, Zhang X, Solano R, Jacobsen SE, Gutierrez C. Genome-wide mapping of Arabidopsis thaliana origins of DNA replication and their associated epigenetic marks. Nature Structural & Molecular Biology. 2011;18:395–400. doi: 10.1038/nsmb.1988. - DOI - PMC - PubMed
- Gernand D, Rutten T, Varshney A, Rubtsova M, Prodanovic S, Brüss C, Kumlehn J, Matzk F, Houben A. Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. The Plant Cell. 2005;17:2431–2438. doi: 10.1105/tpc.105.034249. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD010786/OD/NIH HHS/United States
- S10 RR027303/RR/NCRR NIH HHS/United States
- S10 RR029668/RR/NCRR NIH HHS/United States
- GBMF3068/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials