Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract - PubMed (original) (raw)
. 2015 May 19;42(5):965-76.
doi: 10.1016/j.immuni.2015.04.019.
Elizabeth H Byrne 2, Kathleen E Doherty 2, Brittany A Bowman 2, Hidemi S Yamamoto 3, Magali Soumillon 4, Nikita Padavattan 5, Nasreen Ismail 5, Amber Moodley 6, Mary E Sabatini 7, Musie S Ghebremichael 2, Chad Nusbaum 4, Curtis Huttenhower 8, Herbert W Virgin 9, Thumbi Ndung'u 10, Krista L Dong 11, Bruce D Walker 12, Raina N Fichorova 3, Douglas S Kwon 13
Affiliations
- PMID: 25992865
- PMCID: PMC4461369
- DOI: 10.1016/j.immuni.2015.04.019
Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract
Melis N Anahtar et al. Immunity. 2015.
Abstract
Colonization by Lactobacillus in the female genital tract is thought to be critical for maintaining genital health. However, little is known about how genital microbiota influence host immune function and modulate disease susceptibility. We studied a cohort of asymptomatic young South African women and found that the majority of participants had genital communities with low Lactobacillus abundance and high ecological diversity. High-diversity communities strongly correlated with genital pro-inflammatory cytokine concentrations in both cross-sectional and longitudinal analyses. Transcriptional profiling suggested that genital antigen-presenting cells sense gram-negative bacterial products in situ via Toll-like receptor 4 signaling, contributing to genital inflammation through activation of the NF-κB signaling pathway and recruitment of lymphocytes by chemokine production. Our study proposes a mechanism by which cervicovaginal microbiota impact genital inflammation and thereby might affect a woman's reproductive health, including her risk of acquiring HIV.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1
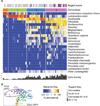
16S rRNA sequencing analysis of cervical swabs reveals low Lactobacillus abundance and four distinct bacterial community structures. (A) Heatmap of bacterial taxa identified by 16S V4 sequencing of cervical swabs collected from 94 women. Cervicotypes (CTs) were determined based on the dominant species: Lactobacillus crispatus (CT1), L. iners (CT2), Gardnerella (CT3), and mixed microflora containing Prevotella (CT4). Nugent scores and bacterial alpha diversity are also shown. (B) Principal coordinates analysis using the weighted UniFrac distance metric on the same 94 samples, colored by CT. See also Figure S1 and Table S1.
Figure 2
16S V4 sequencing and whole-genome shotgun sequencing (WGS) identify very similar bacterial abundances for all CTs, with higher resolution taxonomic identification provided by WGS. Participant #6 represents CT1 (A), #1 represents CT2 (B), #7 represents CT3 (C), #11 represents CT4 (D). See also Figure S2.
Figure 3
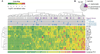
Asymptomatic women display a broad range of baseline genital inflammation that is not explained by STIs. (A) Heatmap of cervicovaginal lavage cytokines from 146 women, each represented by a column. Nugent scores and active STIs (blue: C. trachomatis, N. gonorrhoea, T. vaginalis, and/or HSV-2 positive; gray: negative for that STI) are also displayed. Principal component (PC) analysis was performed on the normalized cytokine concentrations and the first PC (PC1) explained 41% of variation. (B) Pie chart of the STI prevalence in women in the highest quartile of inflammation (n=35). See also Figure S3 and Table S2.
Figure 4
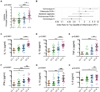
Genital pro-inflammatory cytokine levels vary significantly with microbial community structure and most strongly correlate with CT4 bacterial communities. (A) Cytokine PC1 values from women with bacterial communities CT1–4. (B) Odds ratios and 95% confidence intervals representing the likelihood that a woman with cervicotype 4, an active chlamydia infection, bacterial vaginosis (Nugent score >7), an active Trichomonas infection, or cervicotype 3 is within the top quartile of proinflammatory cytokine levels (as determined by cytokine PC1) versus below the 75th percentile. (C–H) Cervicovaginal lavage IL-1α, IL-1β, TNF-α, IFN-γ, IL-10, and IL-8 concentrations in women with bacterial communities CT1–4. Median and IQR shown. Significance levels were determined by a Kruskal-Wallis test and asterisks denote post-test significance level (* p < 0.05; ** p < 0.01; *** p < 0.001; n=94). See also Figure S4.
Figure 5
Intraindividual longitudinal genital microbiome changes correlate with pro-inflammatory cytokine levels. (A) Cervicovaginal IL-1α, IL-1β, and TNF-α concentrations with bacterial taxa identified by 16S sequencing from matched longitudinal cervical swabs and CVLs collected from two representative participants, #13 and #14. (B–C) Cervicovaginal IL-1α, IL-1β, and TNF-α concentrations from serial time points with either no change in CT (B) or a change (C) (one-tailed Wilcoxon matched pairs test). The order of time points was standardized such that the lower cervicotype was entered as the first time point and the higher cervicotype was entered as the second time point, regardless of the actual chronological order. Any transition, e.g. from CT1 to CT2 and from CT3 to CT4, was considered to be a CT change. See also Figure S5 and Table S3.
Figure 6
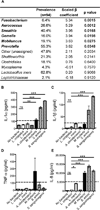
Bacterial species highly correlated with pro-inflammatory cytokines in vivo also stimulate vaginal epithelial cells to produce the same cytokines in vitro. (A) Ridge regression analysis with cytokine PC1 as the response variable and bacterial abundances as the predictor variables. The bacterial prevalence amongst the 94 subjects is also shown; taxa must have an abundance of at least 1% within a sample to be considered present. (B–E) IL-1α, IL-1β, TNF-α, and IL-8 secretion by vaginal epithelial cells after in vitro application of 15 log10 CFU/cm2 of each bacterial species. MALP-2 (a TLR 2/4 agonist; 25nM) treatment was used as a positive control (value denoted by dashed line). P values were determined by a Dunnett test, compared to Lactobacillus crispatus. Data shown as mean and SEM, with two biological replicates. See also Figure S6 and Table S4.
Figure 7
Transcriptional profiling suggests that CT4 bacterial products are sensed by cervical antigen presenting cells (APCs), which contribute to genital inflammation by producing a myriad of pro-inflammatory cytokines and critical T cell chemoattractants. (A, B) CD14 (A) and CD11c (B) staining of endocervical tissue. Scale bar: 50 µm. (C) Heatmap of genes differentially expressed in cervical APCs from women with Lactobacillus dominant communities (>95% abundance) (from left to right: 2 in CT1, 3 in CT2) versus those with high Prevotella abundance (>25%) (n=5 in CT4). Women with STIs were excluded. Only genes with a false discovery rate < 0.05 are shown. Gene set enrichment analysis was used to determine statistically significant similarities with annotated gene sets. (D) Number of live endocervical CD38+ HLADR+ CCR5+ CD4 T cells from women in the highest and lowest quintile of cytokine PC1 (Mann-Whitney, n=16 per group). See also Figure S7 and Table S5.
Comment in
- Cervicovaginal microbiota: simple is better.
Gopinath S, Iwasaki A. Gopinath S, et al. Immunity. 2015 May 19;42(5):790-1. doi: 10.1016/j.immuni.2015.05.006. Immunity. 2015. PMID: 25992855
Similar articles
- Defining characteristics of genital health in South African adolescent girls and young women at high risk for HIV infection.
Dabee S, Barnabas SL, Lennard KS, Jaumdally SZ, Gamieldien H, Balle C, Happel AU, Murugan BD, Williamson AL, Mkhize N, Dietrich J, Lewis DA, Chiodi F, Hope TJ, Shattock R, Gray G, Bekker LG, Jaspan HB, Passmore JS. Dabee S, et al. PLoS One. 2019 Apr 4;14(4):e0213975. doi: 10.1371/journal.pone.0213975. eCollection 2019. PLoS One. 2019. PMID: 30947260 Free PMC article. - Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota.
Manhanzva MT, Abrahams AG, Gamieldien H, Froissart R, Jaspan H, Jaumdally SZ, Barnabas SL, Dabee S, Bekker LG, Gray G, Passmore JS, Masson L. Manhanzva MT, et al. Sci Rep. 2020 Apr 10;10(1):6196. doi: 10.1038/s41598-020-62184-8. Sci Rep. 2020. PMID: 32277092 Free PMC article. - Cervicovaginal microbiota: simple is better.
Gopinath S, Iwasaki A. Gopinath S, et al. Immunity. 2015 May 19;42(5):790-1. doi: 10.1016/j.immuni.2015.05.006. Immunity. 2015. PMID: 25992855 - The Influence of Cervicovaginal Microbiota on Mucosal Immunity and Prophylaxis in the Battle against HIV.
Farcasanu M, Kwon DS. Farcasanu M, et al. Curr HIV/AIDS Rep. 2018 Feb;15(1):30-38. doi: 10.1007/s11904-018-0380-5. Curr HIV/AIDS Rep. 2018. PMID: 29516267 Review. - The barrier to HIV transmission provided by genital tract Lactobacillus colonization.
Mirmonsef P, Spear GT. Mirmonsef P, et al. Am J Reprod Immunol. 2014 Jun;71(6):531-6. doi: 10.1111/aji.12232. Epub 2014 Mar 24. Am J Reprod Immunol. 2014. PMID: 24661438 Review.
Cited by
- The relationship between the vaginal and vulvar microbiomes and lichen sclerosus symptoms in post-menopausal women.
Taylor OA, Birse KD, Hill D'J, Knodel S, Noel-Romas L, Myers A, Marino J, Burgener AD, Pope R, Farr Zuend C. Taylor OA, et al. Sci Rep. 2024 Nov 7;14(1):27094. doi: 10.1038/s41598-024-78372-9. Sci Rep. 2024. PMID: 39511372 Free PMC article. - Genital infections in high-risk human papillomavirus positive Paraguayan women aged 30-64 with and without cervical lesions.
Arévalos A, Valenzuela A, Mongelós P, Barrios H, Rodríguez MI, Báez R, Centurión C, Vester J, Soilán A, Ortega M, Meza L, Páez M, Castro A, Cristaldo C, Soskin A, Deluca G, Baena A, Herrero R, Almonte M, Kasamatsu E, Mendoza L; ESTAMPA Paraguayan Study Group. Arévalos A, et al. PLoS One. 2024 Oct 29;19(10):e0312947. doi: 10.1371/journal.pone.0312947. eCollection 2024. PLoS One. 2024. PMID: 39471231 Free PMC article. - Endometriosis specific vaginal microbiota links to urine and serum N-glycome.
MacSharry J, Kovács Z, Xie Y, Adamczyk B, Walsh C, Reidy F, McAuliffe FM, Kilbane MT, Twomey PJ, Rudd PM, Wingfield M, Butler M, van Sinderen D, Glover L, Saldova R. MacSharry J, et al. Sci Rep. 2024 Oct 25;14(1):25372. doi: 10.1038/s41598-024-76125-2. Sci Rep. 2024. PMID: 39455640 Free PMC article. - Glutamine Attenuates Inflammation and Stimulates Amniotic Cell Proliferation in Premature Rupture of Membranes-related in vitro Models.
Xiang X, Zhang L, Li S, Ren Y, Chen D, Liu L. Xiang X, et al. Reprod Sci. 2024 Oct 4. doi: 10.1007/s43032-024-01691-9. Online ahead of print. Reprod Sci. 2024. PMID: 39367233 - The impact of sex on HIV immunopathogenesis and therapeutic interventions.
Mihealsick E, Word A, Scully EP. Mihealsick E, et al. J Clin Invest. 2024 Sep 17;134(18):e180075. doi: 10.1172/JCI180075. J Clin Invest. 2024. PMID: 39286972 Free PMC article. Review.
References
- Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. Defense factors of vaginal lactobacilli. American journal of obstetrics and gynecology. 2001;185:375–379. - PubMed
- Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013a;56:777–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI079085/AI/NIAID NIH HHS/United States
- R01AI067073/AI/NIAID NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- 1R01AI111918/AI/NIAID NIH HHS/United States
- R01 AI111918/AI/NIAID NIH HHS/United States
- Howard Hughes Medical Institute/United States
- T32GM007753/GM/NIGMS NIH HHS/United States
- 5R01AI079085/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources