Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome - PubMed (original) (raw)
doi: 10.1038/ng.3311. Epub 2015 May 25.
Long N Nguyen 2, Hongbo Yang 3, Maha S Zaki 4, Majdi Kara 5, Tawfeg Ben-Omran 6, Naiara Akizu 1, Rasim Ozgur Rosti 1, Basak Rosti 1, Eric Scott 1, Jana Schroth 1, Brett Copeland 1, Keith K Vaux 1, Amaury Cazenave-Gassiot 7, Debra Q Y Quek 2, Bernice H Wong 2, Bryan C Tan 2, Markus R Wenk 7, Murat Gunel 8, Stacey Gabriel 9, Neil C Chi 3, David L Silver 2, Joseph G Gleeson 1
Affiliations
- PMID: 26005868
- PMCID: PMC4547531
- DOI: 10.1038/ng.3311
Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome
Alicia Guemez-Gamboa et al. Nat Genet. 2015 Jul.
Abstract
Docosahexanoic acid (DHA) is the most abundant omega-3 fatty acid in brain, and, although it is considered essential, deficiency has not been linked to disease. Despite the large mass of DHA in phospholipids, the brain does not synthesize it. DHA is imported across the blood-brain barrier (BBB) through the major facilitator superfamily domain-containing 2a (MFSD2A) protein. MFSD2A transports DHA as well as other fatty acids in the form of lysophosphatidylcholine (LPC). We identify two families displaying MFSD2A mutations in conserved residues. Affected individuals exhibited a lethal microcephaly syndrome linked to inadequate uptake of LPC lipids. The MFSD2A mutations impaired transport activity in a cell-based assay. Moreover, when expressed in mfsd2aa-morphant zebrafish, mutants failed to rescue microcephaly, BBB breakdown and lethality. Our results establish a link between transport of DHA and LPCs by MFSD2A and human brain growth and function, presenting the first evidence of monogenic disease related to transport of DHA in humans.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests
Figures
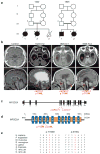
Figure 1. MFSD2A mutations cause severe microcephaly and ventriculomegaly
(a) Consanguineous families 1422 and 1825 designated by number in each generation. Circles: females, squares: males, slashes: deceased, triangle: spontaneous abortion, asterisk: sampled. (b) Upper: axial MRI, lower: parasagittal MRI. Images show enlarged lateral ventricles (asterisks), hypoplasia of the corpus callosum, brain stem (arrow heads) and cerebellum (arrows) in affected children. (c) Exonic structure of MFSD2A with location of the patient mutations. (d) Alignment of amino acid sequences of vertebrate MFSD2A showing the conservation of residues p.T159 and p.S166. (e) Location of mutations relative to predicted protein. TM: transmembrane domains, orange: Major facilitator superfamily, general substrate transporter domain.
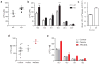
Figure 2. MFSD2A p.T159M and p.S166L mutations display impaired LPC transport
(a, b) Western blot and immunolocalization of MFSD2A (WT), mutant p.T159M, and p.S166L proteins expressed in HEK293 cells. (c–e) Concentration-dependent transport of LPC-[14C]DHA, LPC-[14C]oleate, and LPC [14C]palmitate after 30 min of MFSD2A (WT), mutant p.T159M, and p.S166L proteins expressed in HEK293 cells. (f) Comparison of biological incorporation of radiolabeled LPC-[14C]oleate into phosphatidylcholine (PC). (g, f) Quantification of radiolabeled PC and LPC bands from TLC plates shown in Supplementary Fig 6. (h) View of the internal cavity of human MFSD2A Experiments were performed twice with triplicates. Data are expressed as mean ± SEM. ***p<0.001, ** p <0.01. Specific p values from left to right: p<0.001, p=0.0019.
Figure 3. Zebrafish mfsd2aa morphants present disrupted BBB integrity, lethality and microcephaly
(a) Intracardiac injection of 2000-kD dextran into mfsd2aa morpholino (MO)-injected and control embryos. Arrows: colocalization of dextran (green) and cranial blood vessels (red). Arrowhead: dextran extravasation into the brain parenchyma. (b) Kaplan–Meier plot of mfsd2aa morphants survival (n=457 embryos). (c) mfsd2aa MO (1ng) was co-injected with zebrafish wild-type mfsd2aa mRNA (50ng; n=118), human wild-type MFSD2A mRNA (50ng; n=135), or mutated p.T159M and p.S166L human MFSD2A mRNA (50ng; n=120 and 107 respectively). Bars represent the cumulative % of survival after 1 (dark grey), 2 (light grey) and 3 (black) days post fertilization (dpf). (d) Comparison of the head size of control and mfsd2aa morphants (n=20). *** p <0.001, * p <0.05. Specific p values from left to right: p<0.001, p<0.001, p=0.0143, p=0.0302, p=0.0310.
Figure 4. Total plasma LPC and individual LPC species by lipidomic mass spectrometry
(a,b) Concentration of total plasma LPC and common C16-22 chain lenght LPC species from WT (n=5) and Mfsd2a KO (n=5) mice, with 3 technical replicates. (c) Quantification of injected LPC [14C]oleate over time in the plasma of Mfsd2a KO mice (n=4) realtive to WT (n=3) littermates. (d, e) Total plasma LPC and common LPC species concentrations from age matched controls, unaffected parents and affected individuals from families 1422 and 1825. Analysis was performed once with 3 technical replicates from two independent plasma samples collected on different days. * p <0.05, ** p <0.01, *** p <0.001. Specific p values from left to right: p=0.0094, p=0.0004, p=0.0372, p=0.0002.
Similar articles
- A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome.
Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH. Alakbarzade V, et al. Nat Genet. 2015 Jul;47(7):814-7. doi: 10.1038/ng.3313. Epub 2015 May 25. Nat Genet. 2015. PMID: 26005865 - Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination.
Harel T, Quek DQY, Wong BH, Cazenave-Gassiot A, Wenk MR, Fan H, Berger I, Shmueli D, Shaag A, Silver DL, Elpeleg O, Edvardson S. Harel T, et al. Neurogenetics. 2018 Dec;19(4):227-235. doi: 10.1007/s10048-018-0556-6. Epub 2018 Jul 24. Neurogenetics. 2018. PMID: 30043326 - Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid.
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Nguyen LN, et al. Nature. 2014 May 22;509(7501):503-6. doi: 10.1038/nature13241. Epub 2014 May 14. Nature. 2014. PMID: 24828044 - Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye.
Wong BH, Silver DL. Wong BH, et al. Adv Exp Med Biol. 2020;1276:223-234. doi: 10.1007/978-981-15-6082-8_14. Adv Exp Med Biol. 2020. PMID: 32705603 Review. - Marine Fish-Derived Lysophosphatidylcholine: Properties, Extraction, Quantification, and Brain Health Application.
Ahmmed MK, Hachem M, Ahmmed F, Rashidinejad A, Oz F, Bekhit AA, Carne A, Bekhit AEA. Ahmmed MK, et al. Molecules. 2023 Mar 30;28(7):3088. doi: 10.3390/molecules28073088. Molecules. 2023. PMID: 37049852 Free PMC article. Review.
Cited by
- Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders.
Martinat M, Rossitto M, Di Miceli M, Layé S. Martinat M, et al. Nutrients. 2021 Apr 2;13(4):1185. doi: 10.3390/nu13041185. Nutrients. 2021. PMID: 33918517 Free PMC article. Review. - Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer's Disease.
Sánchez-Campillo M, Ruiz-Pastor MJ, Gázquez A, Marín-Muñoz J, Noguera-Perea F, Ruiz-Alcaraz AJ, Manzanares-Sánchez S, Antúnez C, Larqué E. Sánchez-Campillo M, et al. Int J Mol Sci. 2019 Dec 20;21(1):70. doi: 10.3390/ijms21010070. Int J Mol Sci. 2019. PMID: 31861865 Free PMC article. - Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction.
Lobanova ES, Schuhmann K, Finkelstein S, Lewis TR, Cady MA, Hao Y, Keuthan C, Ash JD, Burns ME, Shevchenko A, Arshavsky VY. Lobanova ES, et al. J Neurosci. 2019 Dec 4;39(49):9689-9701. doi: 10.1523/JNEUROSCI.1142-19.2019. Epub 2019 Nov 1. J Neurosci. 2019. PMID: 31676603 Free PMC article. - Multifaceted Microcephaly-Related Gene MCPH1.
Kristofova M, Ori A, Wang ZQ. Kristofova M, et al. Cells. 2022 Jan 14;11(2):275. doi: 10.3390/cells11020275. Cells. 2022. PMID: 35053391 Free PMC article. Review. - Modulation of the Blood-Brain Barrier for Drug Delivery to Brain.
Han L. Han L. Pharmaceutics. 2021 Nov 27;13(12):2024. doi: 10.3390/pharmaceutics13122024. Pharmaceutics. 2021. PMID: 34959306 Free PMC article. Review.
References
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–579. - PubMed
- Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. - PubMed
- Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. - PubMed
- Engle PL, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- U54HG003067/HG/NHGRI NIH HHS/United States
- S10 OD018521/OD/NIH HHS/United States
- U54HG006504/HG/NHGRI NIH HHS/United States
- K99NS089943/NS/NINDS NIH HHS/United States
- K99 NS089943/NS/NINDS NIH HHS/United States
- R01NS048453/NS/NINDS NIH HHS/United States
- P01 HD070494/HD/NICHD NIH HHS/United States
- U54 HG006504/HG/NHGRI NIH HHS/United States
- R01 NS048453/NS/NINDS NIH HHS/United States
- P01HD070494/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases