mTORC1 signaling activates NRF1 to increase cellular proteasome levels - PubMed (original) (raw)
Review
mTORC1 signaling activates NRF1 to increase cellular proteasome levels
Yinan Zhang et al. Cell Cycle. 2015.
Abstract
Defects in the maintenance of protein homeostasis, or proteostasis, has emerged as an underlying feature of a variety of human pathologies, including aging-related diseases. Proteostasis is achieved through the coordinated action of cellular systems overseeing amino acid availability, mRNA translation, protein folding, secretion, and degradation. The regulation of these distinct systems must be integrated at various points to attain a proper balance. In a recent study, we found that the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) pathway, well known to enhance the protein synthesis capacity of cells while concordantly inhibiting autophagy, promotes the production of more proteasomes. Activation of mTORC1 genetically, through loss of the tuberous sclerosis complex (TSC) tumor suppressors, or physiologically, through growth factors or feeding, stimulates a transcriptional program involving the sterol-regulatory element binding protein 1 (SREBP1) and nuclear factor erythroid-derived 2-related factor 1 (NRF1; also known as NFE2L1) transcription factors leading to an increase in cellular proteasome content. As discussed here, our findings suggest that this increase in proteasome levels facilitates both the maintenance of proteostasis and the recovery of amino acids in the face of an increased protein load consequent to mTORC1 activation. We also consider the physiological and pathological implications of this unexpected new downstream branch of mTORC1 signaling.
Keywords: NFE2L1; NRF2; aging; cancer; muscle; neurodegeneration; proteasome; synaptic plasticity.
Figures
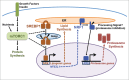
Figure 1.
The mTORC1-SREBP-NRF1-Proteasome pathway. Growth factors and nutrients activate mTORC1, which promotes an increase in cellular protein synthesis. mTORC1 also stimulates activation of the SREBP1 transcription factor by promoting its processing and nuclear accumulation, which requires its trafficking to the Golgi, where it is proteolytically cleaved by 2 proteases, resulting in release of the N-terminus encompassing the mature active transcription factor. Mature SREBP1 binds to SRE sequences in the promoters of genes, including the enzymes of de novo lipid synthesis and NFE2L1, encoding the NRF1 transcription factor. NRF1 is synthesized as an ER transmembrane protein and must be processed to release the active transcription factor. Proteasome inhibitors stimulate this processing, but the nature of the physiological signal is currently unknown. Once activated, NRF1 goes to the nucleus and turns on a subset of genes containing AREs in their promoter, including those encoding all, or nearly all, subunits of the proteasome, leading to an increase in cellular proteasome content. See text for more details.
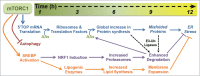
Figure 2.
The temporal response to mTORC1 activation. Within the first hour of mTORC1 activation, it stimulates the translation of 5′TOP mRNAs, inhibits autophagy, and promotes SREBP activation, initiating a cascade of cellular events that promote cell growth while protecting from proteotoxic stress. A key feature of this program is the delayed production of proteasomes by NRF1 induction, which enhances the efficiency of protein degradation following the global increase in protein synthesis, thereby facilitating the clearance of proteins and misfolded proteins that have been independently targeted for degradation by target-specific E3-ubiquitin (Ub) ligases. Downstream of SREBP transcriptional targets, the activation of lipid synthesis and expansion of cellular membranes combined with the NRF1-dependent increase in proteasomes and protein turnover protects cells with activated mTORC1 signaling from proteotoxic stress, including ER stress. The time scale on the top provides a rough estimate of the kinetics of this response, with the precise timing likely to vary in different cell and tissue settings. See text for more details.
Similar articles
- Coordinated regulation of protein synthesis and degradation by mTORC1.
Zhang Y, Nicholatos J, Dreier JR, Ricoult SJ, Widenmaier SB, Hotamisligil GS, Kwiatkowski DJ, Manning BD. Zhang Y, et al. Nature. 2014 Sep 18;513(7518):440-3. doi: 10.1038/nature13492. Epub 2014 Jul 13. Nature. 2014. PMID: 25043031 Free PMC article. - Coordinate regulation of autophagy and the ubiquitin proteasome system by MTOR.
Zhao J, Goldberg AL. Zhao J, et al. Autophagy. 2016 Oct 2;12(10):1967-1970. doi: 10.1080/15548627.2016.1205770. Epub 2016 Jul 26. Autophagy. 2016. PMID: 27459110 Free PMC article. - ER-Resident Transcription Factor Nrf1 Regulates Proteasome Expression and Beyond.
Hamazaki J, Murata S. Hamazaki J, et al. Int J Mol Sci. 2020 May 23;21(10):3683. doi: 10.3390/ijms21103683. Int J Mol Sci. 2020. PMID: 32456207 Free PMC article. Review. - Regulation of mTORC1 by PI3K signaling.
Dibble CC, Cantley LC. Dibble CC, et al. Trends Cell Biol. 2015 Sep;25(9):545-55. doi: 10.1016/j.tcb.2015.06.002. Epub 2015 Jul 6. Trends Cell Biol. 2015. PMID: 26159692 Free PMC article. Review.
Cited by
- The Role of mTOR in Amyotrophic Lateral Sclerosis.
Nogueira-Machado JA, Rocha-Silva F, Gomes NA. Nogueira-Machado JA, et al. Biomedicines. 2025 Apr 13;13(4):952. doi: 10.3390/biomedicines13040952. Biomedicines. 2025. PMID: 40299664 Free PMC article. Review. - Functional Differences between Proteasome Subtypes.
Abi Habib J, Lesenfants J, Vigneron N, Van den Eynde BJ. Abi Habib J, et al. Cells. 2022 Jan 26;11(3):421. doi: 10.3390/cells11030421. Cells. 2022. PMID: 35159231 Free PMC article. Review. - NFE2L1 restrains ferroptosis by transcriptionally regulating HJURP and participates in the progress of oral squamous cell carcinoma.
Zhang M, Wang Z, Yang G, Han L, Wang X. Zhang M, et al. J Bioenerg Biomembr. 2023 Dec;55(6):467-478. doi: 10.1007/s10863-023-09987-2. Epub 2023 Oct 18. J Bioenerg Biomembr. 2023. PMID: 37848756 - The Autophagoproteasome a Novel Cell Clearing Organelle in Baseline and Stimulated Conditions.
Lenzi P, Lazzeri G, Biagioni F, Busceti CL, Gambardella S, Salvetti A, Fornai F. Lenzi P, et al. Front Neuroanat. 2016 Jul 21;10:78. doi: 10.3389/fnana.2016.00078. eCollection 2016. Front Neuroanat. 2016. PMID: 27493626 Free PMC article. - A Tale of Two Proteolytic Machines: Matrix Metalloproteinases and the Ubiquitin-Proteasome System in Pulmonary Fibrosis.
Roque W, Boni A, Martinez-Manzano J, Romero F. Roque W, et al. Int J Mol Sci. 2020 May 29;21(11):3878. doi: 10.3390/ijms21113878. Int J Mol Sci. 2020. PMID: 32485920 Free PMC article. Review.
References
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 2013; 15:555-64; PMID:23728461; http://dx.doi.org/10.1038/ncb2763 - DOI - PMC - PubMed
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307:1098-101; PMID:15718470; http://dx.doi.org/10.1126/science.1106148 - DOI - PubMed
- Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans 2013; 41:906-12; PMID:23863154; http://dx.doi.org/10.1042/BST20130041 - DOI - PubMed
- Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab 2011; 13:495-504; PMID:21531332; http://dx.doi.org/10.1016/j.cmet.2011.04.004 - DOI - PMC - PubMed
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009. 10:307-18; PMID:19339977; http://dx.doi.org/10.1038/nrm2672 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous