Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells - PubMed (original) (raw)
Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells
Elena Vecino et al. PLoS One. 2015.
Abstract
Purpose: Retinal ganglion cells (RGCs) are exposed to injury in a variety of optic nerve diseases including glaucoma. However, not all cells respond in the same way to damage and the capacity of individual RGCs to survive or regenerate is variable. In order to elucidate factors that may be important for RGC survival and regeneration we have focussed on the extracellular matrix (ECM) and RGC integrin expression. Our specific questions were: (1) Do adult RGCs express particular sets of integrins in vitro and in vivo? (2) Can the nature of the ECM influence the expression of different integrins? (3) Can the nature of the ECM affect the survival of the cells and the length or branching complexity of their neurites?
Methods: Primary RGC cultures from adult rat retina were placed on glass coverslips treated with different substrates: Poly-L-Lysine (PL), or PL plus laminin (L), collagen I (CI), collagen IV (CIV) or fibronectin (F). After 10 days in culture, we performed double immunostaining with an antibody against βIII-Tubulin to identify the RGCs, and antibodies against the integrin subunits: αV, α1, α3, α5, β1 or β3. The number of adhering and surviving cells, the number and length of the neurites and the expression of the integrin subunits on the different substrates were analysed.
Results: PL and L were associated with the greatest survival of RGCs while CI provided the least favourable conditions. The type of substrate affected the number and length of neurites. L stimulated the longest growth. We found at least three different types of RGCs in terms of their capacity to regenerate and extend neurites. The different combinations of integrins expressed by the cells growing on different substrata suggest that RGCs expressed predominantly α1β1 or α3β1 on L, α1β1 on CI and CIV, and α5β3 on F. The activity of the integrins was demonstrated by the phosphorylation of focal adhesion kinase (FAK).
Conclusions: Adult rat RGCs can survive and grow in the presence of different ECM tested. Further studies should be done to elucidate the different molecular characteristics of the RGCs subtypes in order to understand the possible different sensitivity of different RGCs to damage in diseases like glaucoma in which not all RGCs die at the same time.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
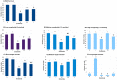
Fig 1. Influence of the substrata and cell distribution.
In all cases the statistical difference was (p<0.05). A: Influence of the substrata on cell survival. The influence of the substrate on the number of observed cells PL and L (•) show significant differences compared to the other substrates; CI ($) shows significant differences in the number of cells compared to the other substrates and CIV and F (#) have significant differences compared to the other substrates B: Distribution of RGC complexity (number of neurites per cell). B1: Low level of complexity. Significant differences were found for PL and L (•) against the rest of the substrates; CI ($) against the rest of substrates and CIV and F (#) with the rest of the substrates. B2: Medium level of complexity. Significant differences were found for each substrate compared to the other substrates. B3: High level of complexity. Significant differences were found for PL, L and CIV (•) against CI and F; CI ($) and F (#) was significantly different to all other substrates. C: Distribution of RGC neurites length between the different substrates. C1: Short length RGCs growing in PL and L (•) are significantly different to the other substrates; and the rest of the RGCs growing in CI ($), CIV (#), or F (##) each was different to the other substrates. C2 Medium length. CI ($) was significantly different to the other substrates, but there was no difference between them PL, L, CIV and F (•). C3 Long length neurites were more frequent when RGCs were plated on L (••) and this difference was significant compared to the other substrates. PL and F (•) were the second substrate in which long neurites were present; CI and CIV had significantly fewer long neurites.
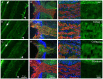
Fig 2. Extracellular matrix distribution in the retina and optic nerve.
Distribution of laminin (A,B,C), collagen I (D,E,F), collagen IV (G,H,I) and fibronectin (J,K,L) in green; in the retina (A,D,G,J scale for all pictures in J) the arrowheads point to the inner limiting membrane while arrows point to outer limiting membrane. The optic nerve head (B, E, H, K scale for all pictures in K) and in the optic nerve (C,F,I,L, scale for all pictures in L). βIII-tubulin labelling in the retinal ganglion cell axons is depicted in red, and DAPI labelling to stain cell nuclei is in blue.
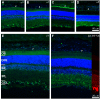
Fig 3. Integrin distribution within the retina.
The images show the integrin label in green, βIII-Tubulin in red and DAPI staining in blue. Distribution of αV, α1, α3, α5 in A, B,C,D (scale bar in D for all α) and labelling for β1 and β3 in E, F (scale bar in F for both β). Arrowheads point to the retinal pigment epithelium. The α integrins and β1 were mainly located in the retinal ganglion cell layer, endothelium of the vessels located closed to the RGCs wile β3 was found mainly within the inner plexiform layer. The retina layers are indicated in E. Outer segments (OS), outer nuclear layer (ONL), inner nuclear layer (INL), inner plexiform layer (IPL) and ganglion cell layer (GCL).
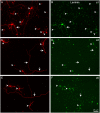
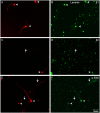
Fig 4. Distribution of α integrins in RGCs growing in laminin.
Paired images A-B, C-D, and E-F of RGCs labelled with a β III-tubulin antibody in red (A, C, E) and for different integrins α1 (B), α3 (D) and α5 (F) in green. Arrowheads point to the cell body of the RGCs while the arrows point to the neurites. Note that the central arrow in C of α3 integrin antibody does not label the long neurite pointed out in D. Scale bar for all pictures in F.
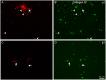
Fig 5. Distribution of β integrins and pY397 FAK in RGCs growing in laminin.
Paired images A-B, C-D, and E-F of RGCs labelled with a βIII-tubulin antibody in red (A,C,E), and β1 (B) and β3 (D) integrins and pY397 FAK (F) in green. Note that the long RGC neurites pointed out with arrows in C and E are not labelled with either β3 or pY397 FAK while the rest of RGCs and their neurites expressed the integrins and were labelled for phosphorylated FAK. Scale bar for all pictures in F.
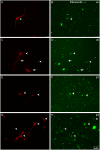
Fig 6. Distribution of α1 and β1 integrins in RGCs growing in collagen IV.
Paired images A-B and C-D of RGCs labelled with a βIII-tubulin antibody (A,C) in red and for α1 (B) and α1 (D) integrins in green. Note that all RGC cell bodies (arrowheads) as well as the neurites (arrows) are labelled with both integrins. Scale bar for all pictures in D.
Fig 7. Distribution of αV, α5, β1 and β3 integrins in RGCs growing in fibronectin.
Paired images A-B, C-D, E-F and G-H of RGCs labelled in red with a βIII-tubulin antibody (A, C, E, G) and αV (B), α5 (D), β1 (F) and β3 (H) integrins are visualised in green. Note that αV was not expressed in RGCs; only a very weak stain can be distinguished while the rest of integrins were expressed in the cell bodies (arrowheads) as well as in the neurites (arrows). Scale bar for all pictures in H.
Similar articles
- β1 integrin-focal adhesion kinase (FAK) signaling modulates retinal ganglion cell (RGC) survival.
Santos AR, Corredor RG, Obeso BA, Trakhtenberg EF, Wang Y, Ponmattam J, Dvoriantchikova G, Ivanov D, Shestopalov VI, Goldberg JL, Fini ME, Bajenaru ML. Santos AR, et al. PLoS One. 2012;7(10):e48332. doi: 10.1371/journal.pone.0048332. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118988 Free PMC article. - MMP Inhibition Preserves Integrin Ligation and FAK Activation to Induce Survival and Regeneration in RGCs Following Optic Nerve Damage.
D'Onofrio PM, Shabanzadeh AP, Choi BK, Bähr M, Koeberle PD. D'Onofrio PM, et al. Invest Ophthalmol Vis Sci. 2019 Feb 1;60(2):634-649. doi: 10.1167/iovs.18-25257. Invest Ophthalmol Vis Sci. 2019. PMID: 30743263 - Integrin-mediated interactions with extracellular matrix proteins for nucleus pulposus cells of the human intervertebral disc.
Bridgen DT, Gilchrist CL, Richardson WJ, Isaacs RE, Brown CR, Yang KL, Chen J, Setton LA. Bridgen DT, et al. J Orthop Res. 2013 Oct;31(10):1661-7. doi: 10.1002/jor.22395. Epub 2013 Jun 4. J Orthop Res. 2013. PMID: 23737292 Free PMC article. - Immunolocalization of integrins in the normal lung and in pulmonary carcinomas.
Koukoulis GK, Warren WH, Virtanen I, Gould VE. Koukoulis GK, et al. Hum Pathol. 1997 Sep;28(9):1018-25. doi: 10.1016/s0046-8177(97)90054-x. Hum Pathol. 1997. PMID: 9308725 - Towards understanding the messengers of extracellular space: Computational models of outside-in integrin reaction networks.
Karagöz Z, Rijns L, Dankers PYW, van Griensven M, Carlier A. Karagöz Z, et al. Comput Struct Biotechnol J. 2020 Dec 29;19:303-314. doi: 10.1016/j.csbj.2020.12.025. eCollection 2021. Comput Struct Biotechnol J. 2020. PMID: 33425258 Free PMC article. Review.
Cited by
- The mechanics of the retina: Müller glia role on retinal extracellular matrix and modelling.
Prieto-López L, Pereiro X, Vecino E. Prieto-López L, et al. Front Med (Lausanne). 2024 Sep 4;11:1393057. doi: 10.3389/fmed.2024.1393057. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39296899 Free PMC article. Review. - Müller glial cells located in the peripheral retina are more susceptible to high pressure: implications for glaucoma.
Pereiro X, Ruzafa N, Azkargorta M, Elortza F, Acera A, Ambrósio AF, Santiago AR, Vecino E. Pereiro X, et al. Cell Biosci. 2024 Jan 5;14(1):5. doi: 10.1186/s13578-023-01186-1. Cell Biosci. 2024. PMID: 38183095 Free PMC article. - Retinal ganglion cell repopulation for vision restoration in optic neuropathy: a roadmap from the RReSTORe Consortium.
Soucy JR, Aguzzi EA, Cho J, Gilhooley MJ, Keuthan C, Luo Z, Monavarfeshani A, Saleem MA, Wang XW, Wohlschlegel J; RReSTORe Consortium; Baranov P, Di Polo A, Fortune B, Gokoffski KK, Goldberg JL, Guido W, Kolodkin AL, Mason CA, Ou Y, Reh TA, Ross AG, Samuels BC, Welsbie D, Zack DJ, Johnson TV. Soucy JR, et al. Mol Neurodegener. 2023 Sep 21;18(1):64. doi: 10.1186/s13024-023-00655-y. Mol Neurodegener. 2023. PMID: 37735444 Free PMC article. Review. - Purified regenerating retinal neurons reveal regulatory role of DNA methylation-mediated Na+/K+-ATPase in murine axon regeneration.
Rizk E, Madrid A, Koueik J, Sun D, Stewart K, Chen D, Luo S, Hong F, Papale LA, Hariharan N, Alisch RS, Iskandar BJ. Rizk E, et al. Commun Biol. 2023 Jan 30;6(1):120. doi: 10.1038/s42003-023-04463-4. Commun Biol. 2023. PMID: 36717618 Free PMC article. - Proteomic Analysis of Retinal Tissue in an S100B Autoimmune Glaucoma Model.
Reinehr S, Guntermann A, Theile J, Benning L, Grotegut P, Kuehn S, Serschnitzki B, Dick HB, Marcus K, Joachim SC, May C. Reinehr S, et al. Biology (Basel). 2021 Dec 23;11(1):16. doi: 10.3390/biology11010016. Biology (Basel). 2021. PMID: 35053014 Free PMC article.
References
- Mey J, Thanos S (1993) Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 602: 304–317. - PubMed
- Glovinsky Y, Quigley HA, Drum B, Bissett RA, Jampel HD (1992) A whole-field scotopic retinal sensitivity test for the detection of early glaucoma damage. Arch Ophthalmol 110: 486–490. - PubMed
- Quigley HA, Sanchez RM, Dunkelberger GR, L'Hernault NL, Baginski TA (1987) Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci 28: 913–920. - PubMed
- Ruiz-Ederra J, Garcia M, Hicks D, Vecino E (2004) Comparative study of the three neurofilament subunits within pig and human retinal ganglion cells. Mol Vis 10: 83–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous