Programmable probiotics for detection of cancer in urine - PubMed (original) (raw)
Programmable probiotics for detection of cancer in urine
Tal Danino et al. Sci Transl Med. 2015.
Abstract
Rapid advances in the forward engineering of genetic circuitry in living cells has positioned synthetic biology as a potential means to solve numerous biomedical problems, including disease diagnosis and therapy. One challenge in exploiting synthetic biology for translational applications is to engineer microbes that are well tolerated by patients and seamlessly integrate with existing clinical methods. We use the safe and widely used probiotic Escherichia coli Nissle 1917 to develop an orally administered diagnostic that can noninvasively indicate the presence of liver metastasis by producing easily detectable signals in urine. Our microbial diagnostic generated a high-contrast urine signal through selective expansion in liver metastases (10(6)-fold enrichment) and high expression of a lacZ reporter maintained by engineering a stable plasmid system. The lacZ reporter cleaves a substrate to produce a small molecule that can be detected in urine. E. coli Nissle 1917 robustly colonized tumor tissue in rodent models of liver metastasis after oral delivery but did not colonize healthy organs or fibrotic liver tissue. We saw no deleterious health effects on the mice for more than 12 months after oral delivery. Our results demonstrate that probiotics can be programmed to safely and selectively deliver synthetic gene circuits to diseased tissue microenvironments in vivo.
Copyright © 2015, American Association for the Advancement of Science.
Figures
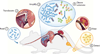
Fig. 1. PROP-Z probiotics for noninvasive cancer detection
The PROP-Z diagnostic platform is made up of probiotic EcN bacteria transformed with a dual-stabilized, high-expression lacZ vector as well as a genomically integrated luxCDABE cassette that allows for luminescent visualization without providing exogenous luciferin (blue). (1) PROP-Z is delivered orally. (2) Probiotics rapidly (within 24 hours) translocate across the gastrointestinal tract and (3) specifically amplify within metastatic tumors present in the liver. (4) PROP-Z expresses high levels of the enzyme lacZ (red), which can cleave systemically injected, cleavable substrates (green and yellow). Cleavage products of the substrates (yellow) filter through the renal system (5) into the urine for detection (6).
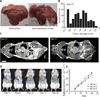
Fig. 2. Colonization of liver metastases by orally administered probiotics
(A) Representative healthy and tumor-bearing livers from Balb/c mice, excised ~21 days after intrasplenic injection of MC26-LucF cells bearing a firefly luciferase transgene. Arrow, small tumor nodules typical of those arising in this metastasis model. Scale bar, 5 mm. (B) Frequency distribution of tumor diameters in mice carrying MC26-LucF liver metastases 14 days after injection of the cells as measured by MRI. n = 10 mice, 98 tumors. (C) MRI images of a mouse with MC26-LucF liver metastases 14 and 17 days after injection of MC26-LucF cells. Yellow arrows, metastases. Scale bar, 10 mm. (D) Representative IVIS images for luminescence of MC26-LucF:Balb/c liver metastasis mice monitored over 21 days. (E) Average radiance for three mice shown as a function of time.
Fig. 3. Colonization of liver metastases by orally delivered PROP: Quantitation and specificity
(A) Twenty-four hours after oral delivery of PROP-Luc [5 × 109 colony-forming units (CFU)] to Balb/c mice carrying liver metastases (at 21 days), livers were excised and photographed (left), imaged for bacterial luminescence with IVIS (right), and then soaked in luciferin to visualize tumors with active mammalian MC26-LucF–derived luminescence (middle). Scale bars, 10 mm. (B) Distribution of colonized and uncolonized liver metastases from three mouse livers. Tumor diameters and colonization were determined by overlaying IVIS images, as described in Materials and Methods and fig. S2. (C) PROP-Z present in organs of healthy Balb/c mice (left) or liver metastases–bearing mice (right) after oral administration were quantified by traditional colony counting on erythromycin-LB plates and confirmed by a qPCR-based assay. Data represent mean colony counts from organs from n = 4 mice ± SEM. (D) Mice bearing DDC-induced liver injury (fibrotic damage visualized by a hematoxylin and eosin stain) were gavaged with 5 × 109 CFU PROP-Z. Excised livers were examined by IVIS. (E) Bacteria levels in liver metastases of control, DDC-treated animals, or Balb/c mice treated with MC26-LucF cells, as determined by colony counts (mean ± SEM; n = 5 each).
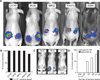
Fig. 4. Colonization in tumor models and different modes of administration
(A) IVIS images showing colonization of subcutaneous human (LS174T-LucF, TOV21G) or mouse (MC26, KBP22,393M1-LucF) tumors by 5 × 106 CFU PROP-Z, 1 to 3 days after intravenous administration to immunocompetent mice. (B) Colonization was determined by observing flank luminescence from bacteria 1 to 3 days after bacteria administration using IVIS and is expressed as % tumors colonized, n = 6 tumors per cell line. (C) IVIS images of mice (MC26-LucF:Balb/c) intravenously injected with 104 or 5 × 106 CFU PROP-Z or administered with 5 × 109 CFU PROP-Z via oral gavage. (D) Colonization determined in two models upon oral (5 × 109) or intravenous (104 to 5 × 106) injection of PROP-Z and expressed as % colonized, n = 6 tumors per dosage.
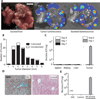
Fig. 5. Dually stabilized vector efficiently maintains PROP-Z activity in vivo
(A) Bacteria luciferase signals from a constitutive luxCDABE circuit (PROP-Luc) or an AHL-inducible luxCDABE circuit (PROPi-Luc) 24 hours after bacterial intravenous injection. (B) The dual-stabilized maintenance system ensures plasmid stability in the tumor environment (C and D) Bacteria were grown overnight with antibiotics (0 hours), and then sub-cultured without antibiotics and assayed for persistence of the lacZ plasmid by performing differential colony counts (black/white colonies on LB S-Gal plates) (see Materials and Methods) (C) and by assaying for lacZ enzymatic activity (D). au, arbitrary unit Mean ± SEM; n = 4. (E) LacZ activity was assayed under reduced nutrient (glucose from 0.02 to 0.002%), pH, and oxygen in the presence of the lacZ inducer (1 mm of IPTG) or in noninducing conditions (0 mM). (F) The plasmid was measured after intravenous delivery of bacteria in a subcutaneous cancer model (LSI 74T-LucF, nude mice). Tumors were analyzed by colony counts on either plasmid-selective (kanamycin + erythromycin) or non–plasmid-selective plates (erythromycin only). Mean ± SEM; n = 5.
Fig. 6. Detection of metastatic tumors by PROP-Z urine diagnostic
(A) Subcutaneous tumor homogenates (nude mice, LS174T-LucF cell line) were analyzed for lacZ activity on days 0,1, and 3 after intravenous injection of 5 × 106 CFU of PROP-Z bacteria (mean ± SEM; n = 5 each). (B) PROP-Z activity was quantified after systemic injection of LuGal, a lacZ substrate that when cleaved produces luciferin, which is measured with a subsequent urine assay for luminescence (mean ± SEM; n = 5,6, and 9). P < 0.05 Student's t test for the +bacteria/+liver metastasis/+lacZ case against the other two cases. (Inset) Representative cleavage assay using a color change substrate (CPRG) for the PROP-Z-treated mice either with or without liver metastases. (C) Paired measurements taken on days 0 and 1 after PROP-Z administered by gavage in individual mice. (Inset) Correlation of luciferase values on days 0 and 1 for each of the n = 10 mice in the cohort. (D) LacZ cleavage ratios shown for the 393M1-LucF liver metastasis model. Mean values ± SEM. P < 0.0001, one-way analysis of variance (ANOVA), Bonferroni post-test resulting in significant (P < 0.05) differences between day 0 versus day 2 and day 1 versus day 2. (Inset) IVIS image showing bacterial colonization of liver metastasis. (E) Urine assay lacZ cleavage data obtained after PROP-Z oral delivery to test animals (+liver metastasis, +PROP-Z), two cohorts of control mice [(M) mock surgery with PBS injected instead of PROP-Z; +liver metastases, +PROP-non-lacZ], and healthy mice (−liver metastases, +PROP-Z). (F) Receiver operating characteristic (ROC) plot illustrates the performance of a binary classifier system as the threshold is varied between case (+LM) and control (−LM) groups in data for (B). The area under the curve (AUC = 0.93) is a metric that characterizes the predictive power of the candidate diagnostic under these specific conditions.
Comment in
- Diagnosis: Programmed probiotics light up liver cancer in urine.
Ray K. Ray K. Nat Rev Gastroenterol Hepatol. 2015 Aug;12(8):429. doi: 10.1038/nrgastro.2015.106. Epub 2015 Jun 16. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26077555 No abstract available.
Similar articles
- Diagnosis: Programmed probiotics light up liver cancer in urine.
Ray K. Ray K. Nat Rev Gastroenterol Hepatol. 2015 Aug;12(8):429. doi: 10.1038/nrgastro.2015.106. Epub 2015 Jun 16. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26077555 No abstract available. - Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice.
Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Stritzker J, et al. Int J Med Microbiol. 2007 Jun;297(3):151-62. doi: 10.1016/j.ijmm.2007.01.008. Epub 2007 Apr 19. Int J Med Microbiol. 2007. PMID: 17448724 - Green fluorescent protein for detection of the probiotic microorganism Escherichia coli strain Nissle 1917 (EcN) in vivo.
Schultz M, Watzl S, Oelschlaeger TA, Rath HC, Göttl C, Lehn N, Schölmerich J, Linde HJ. Schultz M, et al. J Microbiol Methods. 2005 Jun;61(3):389-98. doi: 10.1016/j.mimet.2005.01.007. J Microbiol Methods. 2005. PMID: 15767015 - Drug-inducible remote control of gene expression by probiotic Escherichia coli Nissle 1917 in intestine, tumor and gall bladder of mice.
Loessner H, Leschner S, Endmann A, Westphal K, Wolf K, Kochruebe K, Miloud T, Altenbuchner J, Weiss S. Loessner H, et al. Microbes Infect. 2009 Dec;11(14-15):1097-105. doi: 10.1016/j.micinf.2009.08.002. Epub 2009 Aug 7. Microbes Infect. 2009. PMID: 19665575 - Genetic engineering of probiotic Escherichia coli Nissle 1917 for clinical application.
Ou B, Yang Y, Tham WL, Chen L, Guo J, Zhu G. Ou B, et al. Appl Microbiol Biotechnol. 2016 Oct;100(20):8693-9. doi: 10.1007/s00253-016-7829-5. Epub 2016 Sep 17. Appl Microbiol Biotechnol. 2016. PMID: 27640192 Review.
Cited by
- Spatiotemporal dynamics of distributed synthetic genetic circuits.
Kanakov O, Laptyeva T, Tsimring L, Ivanchenko M. Kanakov O, et al. Physica D. 2016 Apr 1;318-319:116-123. doi: 10.1016/j.physd.2015.10.016. Physica D. 2016. PMID: 26955203 Free PMC article. - Advances in Synthetic Biology and Biosafety Governance.
Li J, Zhao H, Zheng L, An W. Li J, et al. Front Bioeng Biotechnol. 2021 Apr 30;9:598087. doi: 10.3389/fbioe.2021.598087. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33996776 Free PMC article. Review. - In Situ Bioorthogonal Conjugation of Delivered Bacteria with Gut Inhabitants for Enhancing Probiotics Colonization.
Song WF, Yao WQ, Chen QW, Zheng D, Han ZY, Zhang XZ. Song WF, et al. ACS Cent Sci. 2022 Sep 28;8(9):1306-1317. doi: 10.1021/acscentsci.2c00533. Epub 2022 Aug 25. ACS Cent Sci. 2022. PMID: 36188344 Free PMC article. - Achieving Spatial and Molecular Specificity with Ultrasound-Targeted Biomolecular Nanotherapeutics.
Szablowski JO, Bar-Zion A, Shapiro MG. Szablowski JO, et al. Acc Chem Res. 2019 Sep 17;52(9):2427-2434. doi: 10.1021/acs.accounts.9b00277. Epub 2019 Aug 9. Acc Chem Res. 2019. PMID: 31397992 Free PMC article. Review. - Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies.
Chen H, Lei P, Ji H, Yang Q, Peng B, Ma J, Fang Y, Qu L, Li H, Wu W, Jin L, Sun D. Chen H, et al. Mater Today Bio. 2023 Jan 6;18:100543. doi: 10.1016/j.mtbio.2023.100543. eCollection 2023 Feb. Mater Today Bio. 2023. PMID: 36647536 Free PMC article.
References
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. - PubMed
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. - PubMed
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin. Orthop. Relat. Res. 1893:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM069811/GM/NIGMS NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- R01GM69811/GM/NIGMS NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
- P50 GM085764/GM/NIGMS NIH HHS/United States
- P30-ES002109/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials