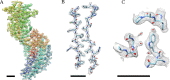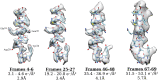Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6 - PubMed (original) (raw)
Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6
Timothy Grant et al. Elife. 2015.
Abstract
Biological specimens suffer radiation damage when imaged in an electron microscope, ultimately limiting the attainable resolution. At a given resolution, an optimal exposure can be defined that maximizes the signal-to-noise ratio in the image. Using a 2.6 Å resolution single particle cryo-EM reconstruction of rotavirus VP6, determined from movies recorded with a total exposure of 100 electrons/Å(2), we obtained accurate measurements of optimal exposure values over a wide range of resolutions. At low and intermediate resolutions, our measured values are considerably higher than obtained previously for crystalline specimens, indicating that both images and movies should be collected with higher exposures than are generally used. We demonstrate a method of using our optimal exposure values to filter movie frames, yielding images with improved contrast that lead to higher resolution reconstructions. This 'high-exposure' technique should benefit cryo-EM work on all types of samples, especially those of relatively low-molecular mass.
Keywords: 20S proteasome; biophysics; high-dose imaging; movie processing; optimal exposure; radiation damage; structural biology; tomography; viruses.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
Figure 1.. (A) Example of an aligned movie sum of the rotavirus double-layered particle (DLP) sample imaged with a total exposure of 100 e−/Å2.
(B) Particle sum created using all frames and (C) the first 3 frames of the movie, demonstrating the kind of data used in the analysis. In all cases, the scale bar represents 500 Å. DOI:
http://dx.doi.org/10.7554/eLife.06980.003
Figure 2.. (A) Density of an isolated VP6 subunit is shown as a mesh along with the docked atomic model.
The model is colored using a rainbow spectrum, starting with the N-terminus in blue and ending with the C-terminus in red. (B) Zoomed region of the VP6 subunit. (C) At higher thresholds, small density features become visible that we interpret as water molecules because their locations are very close to water molecules found in the VP6 crystal structure (Mathieu et al., 2001). A B-factor of −175 Å2 was applied to the DLP reconstruction before 13-fold non-icosahedral averaging to sharpen the VP6 map. In all cases, the scale bar represents 10 Å. DOI:
http://dx.doi.org/10.7554/eLife.06980.004
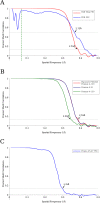
Figure 3.. (A) Fourier Shell Correlation (FSC) curves estimating the resolution of the final VP6 reconstruction calculated using exposure-filtered data.
Two FSC curves were obtained, one from maps calculated from two halves of the data set, another using the modeled atomic coordinates. The dashed green line represents 15 Å, the upper resolution limit used during parameter refinement. (B) FSC curves between half data set reconstructions of the VP6 subunit when using an exposure-filtered sum of frames 4–130, an unfiltered sum of frames 4–130, and an unfiltered sum of frames 4–21, which were determined to be the best set of unfiltered frames by trial and error. Exposure filtering was applied only to the final reconstruction, and not during refinement (C) FSC curve for the reconstruction using only frames 25–27, indicating a resolution of ∼3.4 Å after a pre-exposure of ∼19 e−/Å2. DOI:
http://dx.doi.org/10.7554/eLife.06980.005
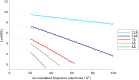
Figure 4.. Example plots of ln(SNR) vs accumulated exposure with associated linear fits at a number of different resolutions.
Data used in this study are shown in darker color, while data for early frames excluded from the analysis due to specimen movement are shown in lighter color. The slopes of the lines become steeper as the resolution increases, corresponding to faster fading of the signal. The linear plots fit the data well, suggesting that in the analyzed regions a single-exponential process is dominant in the decay. DOI:
http://dx.doi.org/10.7554/eLife.06980.006
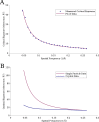
Figure 5.. (A) The measured critical exposure curve plotting the critical exposure at each measured resolution and the fit function for comparison.
(B) Curve plotting the optimal exposure obtained in this study alongside that obtained in a previous study on crystalline specimens, scaled by a factor of 1.25 to compensate for the fact that the previous study was conducted at 200 kV (Baker et al., 2010). DOI:
http://dx.doi.org/10.7554/eLife.06980.007
Figure 6.. Surface rendering of an isolated small helix from different 3-frame reconstructions shown with the docked model.
Each reconstruction is shown with its exposure range and resolution as calculated from the FSC using the 0.143 cut-off. DOI:
http://dx.doi.org/10.7554/eLife.06980.008
Figure 7.. (A) Comparison of an isolated helix from the previously published reconstruction (Campbell, 2015) on the left, and the reconstruction using exposure-filtered data on the right.
The two maps appear almost identical after scaling the amplitudes using diffmap (
http://grigoriefflab.janelia.org/diffmap
), suggesting that in this case exposure filtering performs as well as the weighting based on B-factors implemented in Relion (Scheres, 2014). (B) Plot of FSC curves for the various proteasome reconstructions. The exposure-filtered reconstruction has a resolution of ∼2.8 Å, matching the resolution previously obtained. (C) Plot of the average particle signal-to-noise ratio (SNR) as a function of resolution. The exposure-filtered particles have equal or higher SNR than the other data sets at all resolutions. (D) Plot of FSC curves from the signal-limited data set. In this case, the exposure-filtered reconstruction is of better quality than those derived from the other data sets. Recalculating the non-filtered reconstructions with particle alignment parameters obtained for the exposure-filtered data set increases the resolution to that of the filtered data set (curves labeled ‘EF Alignment’), demonstrating that the loss in resolution was due to particle misalignments. DOI:
http://dx.doi.org/10.7554/eLife.06980.009
Similar articles
- Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM.
Chen JZ, Settembre EC, Aoki ST, Zhang X, Bellamy AR, Dormitzer PR, Harrison SC, Grigorieff N. Chen JZ, et al. Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10644-8. doi: 10.1073/pnas.0904024106. Epub 2009 Jun 1. Proc Natl Acad Sci U S A. 2009. PMID: 19487668 Free PMC article. - Movies of ice-embedded particles enhance resolution in electron cryo-microscopy.
Campbell MG, Cheng A, Brilot AF, Moeller A, Lyumkis D, Veesler D, Pan J, Harrison SC, Potter CS, Carragher B, Grigorieff N. Campbell MG, et al. Structure. 2012 Nov 7;20(11):1823-8. doi: 10.1016/j.str.2012.08.026. Epub 2012 Sep 27. Structure. 2012. PMID: 23022349 Free PMC article. - Single-protein detection in crowded molecular environments in cryo-EM images.
Rickgauer JP, Grigorieff N, Denk W. Rickgauer JP, et al. Elife. 2017 May 3;6:e25648. doi: 10.7554/eLife.25648. Elife. 2017. PMID: 28467302 Free PMC article. - [A review of automatic particle recognition in Cryo-EM images].
Wu X, Wu X. Wu X, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010 Oct;27(5):1178-82. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010. PMID: 21089695 Review. Chinese. - Obtaining high-resolution images of biological macromolecules by using a cryo-electron microscope with a liquid-helium cooled stage.
Mitsuoka K. Mitsuoka K. Micron. 2011 Feb;42(2):100-6. doi: 10.1016/j.micron.2010.08.006. Epub 2010 Sep 8. Micron. 2011. PMID: 20869255 Review.
Cited by
- Structural and functional insights of the human peroxisomal ABC transporter ALDP.
Jia Y, Zhang Y, Wang W, Lei J, Ying Z, Yang G. Jia Y, et al. Elife. 2022 Nov 14;11:e75039. doi: 10.7554/eLife.75039. Elife. 2022. PMID: 36374178 Free PMC article. - Observation of Bacteriophage Ultrastructure by Cryo-Electron Microscopy.
Cuervo A, Losana P, Carrascosa JL. Cuervo A, et al. Methods Mol Biol. 2024;2734:13-25. doi: 10.1007/978-1-0716-3523-0_2. Methods Mol Biol. 2024. PMID: 38066360 - Cryo-electron tomography related radiation-damage parameters for individual-molecule 3D structure determination.
Xue H, Zhang M, Liu J, Wang J, Ren G. Xue H, et al. Front Chem. 2022 Aug 30;10:889203. doi: 10.3389/fchem.2022.889203. eCollection 2022. Front Chem. 2022. PMID: 36110139 Free PMC article. Review. - Molecular mechanism for direct actin force-sensing by α-catenin.
Mei L, Espinosa de Los Reyes S, Reynolds MJ, Leicher R, Liu S, Alushin GM. Mei L, et al. Elife. 2020 Sep 24;9:e62514. doi: 10.7554/eLife.62514. Elife. 2020. PMID: 32969337 Free PMC article. - Cryo-EM structures of the human surfactant lipid transporter ABCA3.
Xie T, Zhang Z, Yue J, Fang Q, Gong X. Xie T, et al. Sci Adv. 2022 Apr 8;8(14):eabn3727. doi: 10.1126/sciadv.abn3727. Epub 2022 Apr 8. Sci Adv. 2022. PMID: 35394827 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources