PD-1 Blockade in Tumors with Mismatch-Repair Deficiency - PubMed (original) (raw)
Clinical Trial
. 2015 Jun 25;372(26):2509-20.
doi: 10.1056/NEJMoa1500596. Epub 2015 May 30.
Jennifer N Uram, Hao Wang, Bjarne R Bartlett, Holly Kemberling, Aleksandra D Eyring, Andrew D Skora, Brandon S Luber, Nilofer S Azad, Dan Laheru, Barbara Biedrzycki, Ross C Donehower, Atif Zaheer, George A Fisher, Todd S Crocenzi, James J Lee, Steven M Duffy, Richard M Goldberg, Albert de la Chapelle, Minori Koshiji, Feriyl Bhaijee, Thomas Huebner, Ralph H Hruban, Laura D Wood, Nathan Cuka, Drew M Pardoll, Nickolas Papadopoulos, Kenneth W Kinzler, Shibin Zhou, Toby C Cornish, Janis M Taube, Robert A Anders, James R Eshleman, Bert Vogelstein, Luis A Diaz Jr
Affiliations
- PMID: 26028255
- PMCID: PMC4481136
- DOI: 10.1056/NEJMoa1500596
Clinical Trial
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency
Dung T Le et al. N Engl J Med. 2015.
Abstract
Background: Somatic mutations have the potential to encode "non-self" immunogenic antigens. We hypothesized that tumors with a large number of somatic mutations due to mismatch-repair defects may be susceptible to immune checkpoint blockade.
Methods: We conducted a phase 2 study to evaluate the clinical activity of pembrolizumab, an anti-programmed death 1 immune checkpoint inhibitor, in 41 patients with progressive metastatic carcinoma with or without mismatch-repair deficiency. Pembrolizumab was administered intravenously at a dose of 10 mg per kilogram of body weight every 14 days in patients with mismatch repair-deficient colorectal cancers, patients with mismatch repair-proficient colorectal cancers, and patients with mismatch repair-deficient cancers that were not colorectal. The coprimary end points were the immune-related objective response rate and the 20-week immune-related progression-free survival rate.
Results: The immune-related objective response rate and immune-related progression-free survival rate were 40% (4 of 10 patients) and 78% (7 of 9 patients), respectively, for mismatch repair-deficient colorectal cancers and 0% (0 of 18 patients) and 11% (2 of 18 patients) for mismatch repair-proficient colorectal cancers. The median progression-free survival and overall survival were not reached in the cohort with mismatch repair-deficient colorectal cancer but were 2.2 and 5.0 months, respectively, in the cohort with mismatch repair-proficient colorectal cancer (hazard ratio for disease progression or death, 0.10 [P<0.001], and hazard ratio for death, 0.22 [P=0.05]). Patients with mismatch repair-deficient noncolorectal cancer had responses similar to those of patients with mismatch repair-deficient colorectal cancer (immune-related objective response rate, 71% [5 of 7 patients]; immune-related progression-free survival rate, 67% [4 of 6 patients]). Whole-exome sequencing revealed a mean of 1782 somatic mutations per tumor in mismatch repair-deficient tumors, as compared with 73 in mismatch repair-proficient tumors (P=0.007), and high somatic mutation loads were associated with prolonged progression-free survival (P=0.02).
Conclusions: This study showed that mismatch-repair status predicted clinical benefit of immune checkpoint blockade with pembrolizumab. (Funded by Johns Hopkins University and others; ClinicalTrials.gov number, NCT01876511.).
Figures
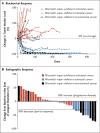
Figure 1. Clinical Responses to Pembrolizumab Treatment
The biochemical responses to pembrolizumab treatment are shown in Panel A. Serum levels of protein biomarkers were measured at the start of each treatment cycle, and the values represent percentage changes from baseline. Each line represents one patient; patients were included if their baseline tumor marker values were higher than the upper limit of normal. CA-125 was used as the biomarker for one patient with endometrial cancer, CA19-9 was used for one patient with cholangiocarcinoma and one patient with ampullary cancer, and carcinoembryonic antigen (CEA) was used for all other patients. Radiographic responses to treatment with pembrolizumab, evaluated on the basis of Response Evaluation Criteria in Solid Tumors (RECIST), are shown in Panel B. Tumor responses were measured at regular intervals, and the values shown are the largest percentage change in the sum of longest diameters from the baseline measurements of each measurable tumor. Each bar represents one patient.
Figure 2. Clinical Benefit of Pembrolizumab Treatment According to Mismatch-Repair Status
Kaplan–Meier curves are shown for progression-free survival in the cohorts with colorectal cancer (Panel A), overall survival in the cohorts with colorectal cancer (Panel B), progression-free survival among patients with mismatch repair–deficient noncolorectal cancers (Panel C), and overall survival among patients with mismatch repair–deficient noncolorectal cancers (Panel D). In both cohorts with mismatch repair–deficient tumors, median overall survival was not reached. Patients in the cohort with mismatch repair–proficient cancers had a median progression-free survival of 2.2 months (95% CI, 1.4 to 2.8) and a median overall survival of 5.0 months (95% CI, 3.0 to not estimable). Patients with mismatch repair–deficient noncolorectal cancers had a median progression-free survival of 5.4 months (95% CI, 3 to not estimable).
Comment in
- PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.
Diaz LA Jr, Le DT. Diaz LA Jr, et al. N Engl J Med. 2015 Nov 12;373(20):1979. doi: 10.1056/NEJMc1510353. N Engl J Med. 2015. PMID: 26559582 No abstract available. - PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.
Asaoka Y, Ijichi H, Koike K. Asaoka Y, et al. N Engl J Med. 2015 Nov 12;373(20):1979. doi: 10.1056/NEJMc1510353. N Engl J Med. 2015. PMID: 26559583 No abstract available. - Case 23-2015: A Woman with Headache, Cognitive Impairment, and Weakness.
Junck L. Junck L. N Engl J Med. 2015 Nov 12;373(20):1983. doi: 10.1056/NEJMc1510498. N Engl J Med. 2015. PMID: 26559591 No abstract available.
Similar articles
- Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer.
André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. André T, et al. N Engl J Med. 2020 Dec 3;383(23):2207-2218. doi: 10.1056/NEJMoa2017699. N Engl J Med. 2020. PMID: 33264544 Clinical Trial. - Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Marabelle A, et al. J Clin Oncol. 2020 Jan 1;38(1):1-10. doi: 10.1200/JCO.19.02105. Epub 2019 Nov 4. J Clin Oncol. 2020. PMID: 31682550 Free PMC article. Clinical Trial. - Programmed death 1 blockade, an Achilles heel for MMR-deficient tumors?
Lin AY, Lin E. Lin AY, et al. J Hematol Oncol. 2015 Nov 5;8:124. doi: 10.1186/s13045-015-0222-5. J Hematol Oncol. 2015. PMID: 26542241 Free PMC article. - Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy.
Zhao P, Li L, Jiang X, Li Q. Zhao P, et al. J Hematol Oncol. 2019 May 31;12(1):54. doi: 10.1186/s13045-019-0738-1. J Hematol Oncol. 2019. PMID: 31151482 Free PMC article. Review. - Clinical Development of Immunotherapy for Deficient Mismatch Repair Colorectal Cancer.
Thomas J, Leal A, Overman MJ. Thomas J, et al. Clin Colorectal Cancer. 2020 Jun;19(2):73-81. doi: 10.1016/j.clcc.2020.02.002. Epub 2020 Feb 10. Clin Colorectal Cancer. 2020. PMID: 32173280 Review.
Cited by
- In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma.
Lurje I, Werner W, Mohr R, Roderburg C, Tacke F, Hammerich L. Lurje I, et al. Front Immunol. 2021 May 7;12:650486. doi: 10.3389/fimmu.2021.650486. eCollection 2021. Front Immunol. 2021. PMID: 34025657 Free PMC article. Review. - CD8+ T cell-associated genes MS4A1 and TNFRSF17 are prognostic markers and inhibit the progression of colon cancer.
Song Y, Zhang Z, Zhang B, Zhang W. Song Y, et al. Front Oncol. 2022 Sep 20;12:941208. doi: 10.3389/fonc.2022.941208. eCollection 2022. Front Oncol. 2022. PMID: 36203424 Free PMC article. - The efficacy and safety of antibodies targeting PD-1 for treatment in advanced esophageal cancer: A systematic review and meta-analysis.
Lu Y, Guan L, Xu M, Wang F. Lu Y, et al. Transl Oncol. 2021 Jun;14(6):101083. doi: 10.1016/j.tranon.2021.101083. Epub 2021 Mar 27. Transl Oncol. 2021. PMID: 33784583 Free PMC article. - CEA as a blood-based biomarker in anal cancer.
Hester R, Advani S, Rashid A, Holliday E, Messick C, Das P, You YN, Taniguchi C, Koay EJ, Bednarski B, Rodriguez-Bigas M, Skibber J, Wolff R, Chang GJ, Minsky BD, Foo WC, Rothschild N, Morris VK, Eng C. Hester R, et al. Oncotarget. 2021 May 25;12(11):1037-1045. doi: 10.18632/oncotarget.27959. eCollection 2021 May 25. Oncotarget. 2021. PMID: 34084278 Free PMC article. - Curcumin: A Novel Way to Improve Quality of Life for Colorectal Cancer Patients?
Layos L, Martínez-Balibrea E, Ruiz de Porras V. Layos L, et al. Int J Mol Sci. 2022 Nov 14;23(22):14058. doi: 10.3390/ijms232214058. Int J Mol Sci. 2022. PMID: 36430537 Free PMC article. Review.
References
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. - PubMed
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. - PubMed
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA57345/CA/NCI NIH HHS/United States
- R01 CA067941/CA/NCI NIH HHS/United States
- R37 CA043460/CA/NCI NIH HHS/United States
- U01 CA067941/CA/NCI NIH HHS/United States
- CA16058/CA/NCI NIH HHS/United States
- P30CA006973/CA/NCI NIH HHS/United States
- P50 CA062924/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- CA67941/CA/NCI NIH HHS/United States
- CA43460/CA/NCI NIH HHS/United States
- CA163672/CA/NCI NIH HHS/United States
- P50CA062924/CA/NCI NIH HHS/United States
- R37 CA057345/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- R01 CA057345/CA/NCI NIH HHS/United States
- K23 CA163672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources