Structural and functional features of central nervous system lymphatic vessels - PubMed (original) (raw)
. 2015 Jul 16;523(7560):337-41.
doi: 10.1038/nature14432. Epub 2015 Jun 1.
Igor Smirnov 1, Timothy J Keyes 1, Jacob D Eccles 2, Sherin J Rouhani 3, J David Peske 3, Noel C Derecki 1, David Castle 4, James W Mandell 5, Kevin S Lee 6, Tajie H Harris 1, Jonathan Kipnis 7
Affiliations
- PMID: 26030524
- PMCID: PMC4506234
- DOI: 10.1038/nature14432
Structural and functional features of central nervous system lymphatic vessels
Antoine Louveau et al. Nature. 2015.
Erratum in
- Corrigendum: Structural and functional features of central nervous system lymphatic vessels.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Louveau A, et al. Nature. 2016 May 12;533(7602):278. doi: 10.1038/nature16999. Epub 2016 Feb 24. Nature. 2016. PMID: 26909581 No abstract available.
Abstract
One of the characteristics of the central nervous system is the lack of a classical lymphatic drainage system. Although it is now accepted that the central nervous system undergoes constant immune surveillance that takes place within the meningeal compartment, the mechanisms governing the entrance and exit of immune cells from the central nervous system remain poorly understood. In searching for T-cell gateways into and out of the meninges, we discovered functional lymphatic vessels lining the dural sinuses. These structures express all of the molecular hallmarks of lymphatic endothelial cells, are able to carry both fluid and immune cells from the cerebrospinal fluid, and are connected to the deep cervical lymph nodes. The unique location of these vessels may have impeded their discovery to date, thereby contributing to the long-held concept of the absence of lymphatic vasculature in the central nervous system. The discovery of the central nervous system lymphatic system may call for a reassessment of basic assumptions in neuroimmunology and sheds new light on the aetiology of neuroinflammatory and neurodegenerative diseases associated with immune system dysfunction.
Conflict of interest statement
All authors declare no financial interests or conflict of interests.
Figures
Extended Figure 1. Meningeal immunity and lymphatic vessels in the dural sinuses
a. Representative image of CD31 staining in whole mount meninges (scale bar = 2,000 μm). b. Representative images of T cells (CD3e, arrowheads) in the dura/arachnoid, pia, dural sinuses, and choroid plexus (scale bar = 70 μm). c. Quantification of T cell density in different meningeal compartments (mean ± SEM; n =6 animals each group; ***p<0.001; Kruskal-Wallis test with Dunn’s post hoc test). d. Quantification of MHCII-expressing cells in different meningeal compartments (mean ± SEM; n = 6 animals each group; ***p<0.001; Kruskal-Wallis test with Dunn’s post hoc test). e. Adult mice were injected i.v. with 100μl of DyLight 488 lectin 5 min prior to sacrifice to enable labeling of the cardiovascular system. Meninges were harvested and stained with anti-CD3e. Representative orthogonal images of T cell localization in the lumen (white arrowhead) and outside of the sinus (yellow arrowhead; n=2 mice; scale bar = 70 μm). f. Adult mice were injected i.v. with 10μg of FITC-conjugated anti-CD45 antibody or FITC-conjugated isotype antibody. Meninges were harvested one hour after the injection and labeled with anti-CD3e. Representative images of CD3e immunolabeling around dural sinuses are shown. CD45 positive cells do not co-localize with CD3+ cells (a), suggesting an abluminal localization of the later (n = 2 mice each group; scale bar = 20 μm). g. Representative 3D reconstruction of the lymphatic vessels localization around the superior sagittal sinus. Adult mice were injected i.v. with 100μl of DyLight 488 lectin 5 min prior to sacrifice in order to stain the cardiovascular system. Meninges were harvested and labeled with anti-Lyve-1. The lack of lectin staining in the Lyve-1-positive meningeal lymphatic vessels suggests that they are independent of the cardiovascular system (n = 3 mice; scale bars = 50 μm and 120 μm). The mounting of the whole meninges results in the flattening of the sinus, thus it does not appear tubular.
Extended Figure 2. Identification, characterization and validation of the expression of classical lymphatic endothelial cell markers by the meningeal lymphatic vessels
a. Representative images of Prox1 labeling on meningeal Lyve-1 expressing vessels (n = 3 mice; scale bar = 10 μm). b. Schematic representation of the whole mount dissection of the diaphragm. c. Characterization of the specificity of the podoplanin antibody. Representative images of whole mount diaphragm labeled with anti-Lyve-1 and anti-podoplanin (ci), control isotype (cii) or the anti-podoplanin pre-incubated overnight with a saturated concentration of recombinant podoplanin protein (ciii; scale bar = 20 μm). d. Characterization of the specificity of the VEGFR3 antibody. Representative images of whole mount diaphragm and dura mater labeled with anti-Lyve-1 and anti-VEGFR3 (di), secondary antibody only (dii), or the anti-VEGFR3 pre-incubated overnight with a saturated concentration of recombinant VEGFR3 protein (diii; scale bar = 20 μm). e. Quantification of the number of Prox1+ nuclei per mm of lymphatic vessel (mean ± SEM; n = 4 animals each group).
Extended Figure 3. Identification of the meningeal lymphatic endothelial cell population by flow cytometry
a. FACS analysis of the lymphatic endothelial cells in diaphragm, skin (ear), and dural sinuses. Gating strategy employed to identify lymphatic endothelial cells (CD31+podoplanin+). Lymphatic endothelial cells are identified as singlet, live cells, CD45 negative and CD31+podoplanin+. b. Representative dot plots for lymphatic endothelial cells (CD31+podoplanin+) in the diaphragm, skin, and dura mater of adult mice.
Extended Figure 4. Pilot identification of lymphatic vessels in human dura
a. Representative image of a formalin-fixed coronal section of human superior sagittal sinus. b–c. Representative images of Lyve-1 staining on coronal section of human superior sagittal sinus (scale bar = 100 μm). The box in c highlights the presence of Lyve-1 expressing macrophages in human meninges, as seen in mice. d. Representative images of Lyve-1 and CD68 staining of coronal sections of human superior sagittal sinus. Note the absence of CD68 positivity on Lyve-1 positive structures (scale bar = 50 μm). e. Representative images of podoplanin and Lyve-1 staining of coronal sections of human superior sagittal sinus (scale bar = 50 μm).
Extended Figure 5. Initial lymphatic features of meningeal lymphatic vessels
a. Representative images of CCL21 and Lyve-1 labeling of the meningeal lymphatic vessels (scale bar = 10 μm). b–c . Representative images of VE-Cadherin and Lyve-1 staining on meningeal blood vessels (b) and meningeal lymphatic vessels (c), arrowheads point to the VE-Cadherin aggregates; scale bar = 10 μm). d–f. Representative images of Claudin-5 and Lyve-1 staining on meningeal blood (d) and lymphatic (e) vessels, and diaphragm lymphatic vessels (f); arrowheads point to Claudin-5 aggregates (scale bar = 10 μm). g–h. Representative images of integrin-α9 and Lyve-1 labeling on skin (g; ear) and meninges whole mount (h). Scale bar = 40 μm. No integrin-α9 expressing valves were detected in the meningeal lymphatic vessels. i. Representative low power micrographs (transmission EM) of the meningeal lymphatic vessels (scale bar = 2 μm); (L = lumen; SC = supporting cell; LEC = lymphatic endothelial cell; BEC = sinusal endothelial cell). Red arrowheads point to anchoring filaments. j. Table summarizing morphological features of the lymphatic network in different regions of the meninges and the diaphragm. Diameters are expressed in μm and branching as number of branches per mm of vessel; (mean ± SEM; n = 4 animals each group, *p<0.05, **p<0.01, ***p<0.001; Two way ANOVA with Bonferroni post-hoc test). For statistics, the presented comparisons were between the diaphragm and the superior sagittal sinus and between the superior sagittal sinus and the transverse sinuses.
Extended Figure 6. Drainage of CSF into the meningeal lymphatic vessels
a. Representative z-stack of QDot655 filled CSF drainage both in the blood vasculature (sinus) and in the meningeal lymphatic vessels after i.c.v. injection (scale bar = 20 μm). b. Representative images of CD31 and Lyve-1 immunostaining on whole mount meninges. Adult mice were injected i.c.v. with 2.5μg of Alexa 488 conjugated anti-Lyve-1 antibody. Thirty minutes after the injection, the meninges were harvested and stained with CD31. Injected in vivo, the Lyve-1 antibody illuminates the lymphatic vessels (scale bar = 20 μm). c. Representative z-stack of superior sagittal sinus of adult mice injected i.v. with QDot655 and i.c.v. with alexa488 conjugated anti-Lyve-1 antibody. ci. Coronal section of the z-stack presented in panel c. The signal from the remaining skull and/or collagen-rich structure above the meninges was recorded (blue). cii. 3D reconstruction of the z-stack presented in panel c showing the localization of the meningeal lymphatic vessels under the skull (scale bar = 50μm).
Extended Figure 7. Meningeal lymphatic vessels carrying immune cells
a. Representative images of T cells (CD3e) and lymphatic endothelial cells (Lyve-1) on dural sinuses (scale bar=20μm). aii–aiii. Orthogonal sections representing T cell localization around (aii) and within (aiii) the Lyve-1 structures (scale bar=5μm). b. Quantification of the sinusal T cells and MHCII-expressing cells within the lymphatic vessels (mean ± SEM, n = 7–8 mice). c–d. Representative images of Lyve-1 staining on dural meninges from CD11cYFP mice (scale bar = 20μm). CD11c-positive cells (most probably dendritic cells) can be found inside the meningeal lymphatic vessels. e. Representative images of B220+ cells and lymphatic endothelial cells (Lyve-1) immunolabeling in the meninges (yellow arrowheads indicate B220+CD11c– cells; scale bar=20 μm). f. Representative dot plots of B220+ cells (gated on singlets, live, CD45+) within the dural sinuses expressing CD19; ~40% of the B220+ cells express CD19.
Extended Figure 8. Drainage of Evans blue from the meningeal lymphatics but not the nasal mucosa into the deep cervical lymph nodes
a–c. Adult mice were injected i.c.v. with 5μl of 10% Evans blue. The meninges were harvested 30 min after injection and Evans blue localization was assessed by confocal microscopy. a. Representative images of Evans blue localization in both the sinus and the meningeal lymphatic vessels (n = 9 mice; scale bar = 40 μm). b. Representative profile of Evans blue and Lyve-1 relative fluorescence intensity on a cross-section of the image presented in panel a. c. Quantification of the average intensity of Evans blue in the sinus, the lymphatic vessels and the meninges of adult mice (mean ± SEM, n = 16 analyzed fields from 4 independent animals; **p<0.01, Kruskall-Wallis with Dunn’s multiple comparisons test). d–e. Adult mice were injected intranasally with 5μl of 10% Evans blue. The successful targeting of the nasal mucosa (d) and the lack of accumulation of Evans blue in the deep cervical lymph nodes (e) 30 min after the injection are demonstrated.
Extended Figure 9. Effects of deep cervical lymph node resection and of the lymphatic vessels ligation on the meningeal immune compartment
a–e. The deep cervical lymph nodes were resected (xDCLN) or sham-operated. Three weeks after resection, the meninges were harvested, single cells isolated, and analyzed for T cell content by flow cytometry. a. Gating strategy to analyze meningeal T cells. Meningeal T cells are selected for singlets, CD45**+, live cells and TCRβ+**. b. Representative dot plot for CD8+ and CD4+ T cells in meninges of sham and xDCLN mice. c. Quantification of total T cells (TCRβ+), CD4+ and CD8+ in the meninges of xDCLN and sham mice (mean ± SEM; n = 3 animals each group; *p=0.018; **p=0.006 (CD8) p=0.003 (TCRb); Student’s T test; a representative experiment, out of two independently performed, is presented). d. Representative expression of CD62L and CD44 by CD4࿕ T cells phenotype in sham and xDCLN mice (n = 3 mice/group). e. Representative histogram for CD71 expression by meningeal CD4࿕ T cells in sham and xDCLN mice (n = 3 mice/group). f. Representative images of the ligation surgery. To highlight the lymph vessels, Evans blue was injected i.c.v. prior to the surgery. Black arrowhead points to the node, yellow arrowhead points to the ligated Evans blue filled vessels. g. Sham-operated or ligated animals were injected i.c.v. with 5μl of 10% Evans blue. The deep cervical lymph nodes were harvested 30 min after the injection and analyzed for Evans blue content. Representative images of the Evans blue accumulation in the deep cervical lymph nodes of the sham-operated and ligated animals are presented. h. Quantification of the meningeal lymphatic vessel diameter in the superior sagittal sinus and the transverse sinuses in sham mice and after ligation of the collecting lymphatic vessels (mean ± SEM, n = 5 mice/group; Two-way ANOVA with Bonferroni post hoc test).
Extended Figure 10. Connection between the glymphatic system and the meningeal lymphatic system
A schematic representation of a connection between the glymphatic system, responsible for collecting of the interstitial fluids from within the CNS parenchyma to CSF, and our newly identified meningeal lymphatic vessels.
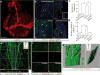
Figure 1. Abluminal distribution of meningeal T cells and identification of Lyve-1 expressing vessels adjacent to the dural sinuses
a. Schematic representation of the whole mount dissection of the dura mater. (SSS: Superior Sagittal Sinus; TS: Transverse Sinus) b. Representative images of CD3e labeling in whole mount meninges (scale bar = 2,000 μm). bii–biii. Higher magnification of the boxes highlighted in bi (scale bar = 90μm (bii) or 150 μm (biii)). c. Schematic representation of a coronal section of whole mount meninges. d. Representative image of a coronal section of whole mount meninges (scale bar = 200μm). e. Representative images of CD3e and CD31 immunolabeling in a coronal section of whole-mount meninges. Scale bar = 100 μm. eii. Higher magnification of the box highlighted in ei (scale bar = 30μm). f. Quantification of the percentage of sinusal T cells localized abluminally vs. luminally to the superior sagittal sinus (mean ± SEM; n = 18 fields analyzed from 3 independent animals; ***p=0.0008, Mann-Whitney test). g. Representative images of CD3e and MHCII-expressing cells around the superior sagittal sinus (meningeal cartoons here and elsewhere depict the location of the presented images; scale bar = 50 μm). gii. Higher magnification of the box highlighted in gi (scale bar = 10 μm). giii. High magnification of CD3 and MHCII-expressing cells (scale bar = 10 μm). h. Representative image of CD31 and CD3e labeling around the superior sagittal sinus (scale bar = 30 μm). i. Quantification of the number of T cells per mm of vessels in the perisinusal CD31+ vessels and in similar diameter meningeal blood vessels (mean ± SEM; n = 3 animals; *p=0.05; One-tailed Mann-Whitney test). j. Representative image of Lyve-1 labeling on whole-mount meninges (scale bar = 1,000 μm). k. Higher magnification of Lyve-1 expressing vessels (scale bar = 70 μm); arrowheads indicate Lyve-1+ macrophages. l. Representative images of CD31 and Lyve-1 labeling of a coronal section of the superior sagittal sinus (scale bar = 70 μm). mi. Higher magnification of a Lyve-1 positive vessel presenting a conduit-like structure (scale bar = 50 μm). mii. Higher magnification of the Lyve-1+ vessel presented in panel mi; arrowhead points to the lumen of the vessel.
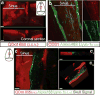
Figure 2. Molecular and structural characterization of meningeal lymphatic vessels
a. Representative images of Prox1 expression in the nuclei of Lyve-1+ vessels in the dural sinuses of Prox1tdT mice (scale bar = 10 μm). b. Representative images of podoplanin and Lyve-1 labeling on dural sinuses (scale bar = 40 μm). c. Representative images of VEGFR3 and Lyve-1 staining on dural sinuses (scale bar = 20 μm). d–e. Adult mice were injected i.c.v. (cisterna magna) with 4μg of rhVEGF-c (Cys156Ser) or with PBS. Meninges were harvested 7 and 14 days after the injection. d. Representative images of Lyve-1 and Prox1 labeling of meninges at day 7 after injection (scale bar = 30μm). e. Quantification of the meningeal lymphatic vessel diameter (mean ± SEM; n = 4 mice each group; *p<0.05 Two-way ANOVA with Bonferroni post-hoc test). f–g. Representative images of smooth muscle cells (alpha-smooth muscle actin; α-SMA) and Lyve-1 labeling on dural sinuses (scale bars = 50μm (g) or 20μm (h)). h. Representative low power micrograph (transmission EM) of a meningeal lymphatic vessel (scale bar = 5 μm); hii. Higher magnification of the box highlighted in hi; yellow arrowheads – basement membrane; red arrowheads – anchoring filaments (collagen fibers); green arrowheads – cellular junction.
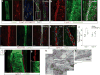
Figure 3. Functional characterization of meningeal lymphatic vessels
a–d. Representative z-stacks of the superior sagittal sinus of adult mice injected intravenously (i.v.) with fluorescein and intracerebroventricularly (i.c.v.) with QDot655 (n = 3 mice). a–b Low magnification images are presented showing fluorescein labeling in a meningeal blood vessel and in the superior sagittal sinus. In contrast, QDot655 labeling is prominent in the perisinusal vessel. c–d. Coronal section of the z-stack presented in panels a and b (scale bar = 20μm c–d). e. Representative z-stack of CSF-filled vessel from a mouse injected i.c.v. with both QDot655 and alexa488-conjugated anti-Lyve-1 antibody (n =3 mice; scale bar = 30μm). fi. Representative image of immunolabeling for CD3e and MHCII along with Lyve-1 in the meninges (scale bar = 15μm). fii. Representative image of a 3D reconstruction of the meningeal lymphatic vessels showing the luminal localization of the CD3e and MHCII-expressing cells (scale bar = 20 μm). g–h. Adult mice were injected i.c.v. with 5μl of 10% Evans blue. Superficial cervical lymph nodes (g) and deep cervical lymph nodes (h) were analyzed 30 min after injection (n = 5 mice); white arrowheads indicate the lymph nodes (g–h); yellow arrowheads indicate the Evans blue filled vessels arising near the internal jugular vein into the deep cervical lymph nodes (h). i–j. The collecting vessels draining into the deep cervical lymph nodes (yellow arrowheads in h) were ligated or sham-operated. Eight hours after the ligation, the meninges were collected and immunolabeled for Lyve-1. Representative images of immunolabeling for Lyve-1 in the transverse sinus of ligated and sham-operated mice (i; scale bar = 30 μm). Dot plots represent measurement of the meningeal lymphatic vessel diameters (j; mean ± SEM; n = 5 mice each group from 2 independent experiments; *p=0.031; Mann-Whitney test).
Comment in
- Neuroimmunology: A brain drain.
Bordon Y. Bordon Y. Nat Rev Immunol. 2015 Jul;15(7):404. doi: 10.1038/nri3878. Epub 2015 Jun 19. Nat Rev Immunol. 2015. PMID: 26089284 No abstract available. - Neuroimmunology: Uncovering the secrets of the 'brain drain'--the CNS lymphatic system is finally revealed.
Wood H. Wood H. Nat Rev Neurol. 2015 Jul;11(7):367. doi: 10.1038/nrneurol.2015.105. Epub 2015 Jun 23. Nat Rev Neurol. 2015. PMID: 26100742 No abstract available. - Neuroanatomy: Forgotten findings of brain lymphatics.
Mezey É, Palkovits M. Mezey É, et al. Nature. 2015 Aug 27;524(7566):415. doi: 10.1038/524415b. Nature. 2015. PMID: 26310754 No abstract available. - Implications of the discovery of brain lymphatic pathways.
Iliff JJ, Goldman SA, Nedergaard M. Iliff JJ, et al. Lancet Neurol. 2015 Oct;14(10):977-9. doi: 10.1016/S1474-4422(15)00221-5. Lancet Neurol. 2015. PMID: 26376966 Free PMC article. No abstract available. - Lymphatic vessels of the dura mater: a new discovery?
Bucchieri F, Farina F, Zummo G, Cappello F. Bucchieri F, et al. J Anat. 2015 Nov;227(5):702-3. doi: 10.1111/joa.12381. Epub 2015 Sep 18. J Anat. 2015. PMID: 26383824 Free PMC article. No abstract available. - Landmark Article Supports Osteopathic Medicine.
Hoegerl C. Hoegerl C. J Am Osteopath Assoc. 2015 Nov;115(11):646. doi: 10.7556/jaoa.2015.134. J Am Osteopath Assoc. 2015. PMID: 26501755 No abstract available. - Landmark Article Supports Osteopathic Medicine? Setting the Record Straight.
Heemstra VL. Heemstra VL. J Am Osteopath Assoc. 2016 Apr;116(4):200. doi: 10.7556/jaoa.2016.040. J Am Osteopath Assoc. 2016. PMID: 27018950 No abstract available. - Response.
Hoegerl C. Hoegerl C. J Am Osteopath Assoc. 2016 Apr;116(4):200. doi: 10.7556/jaoa.2016.041. J Am Osteopath Assoc. 2016. PMID: 27018951 No abstract available.
Similar articles
- [Lymphatic system in central nervous system].
Thomas JL, Jacob L, Boisserand L. Thomas JL, et al. Med Sci (Paris). 2019 Jan;35(1):55-61. doi: 10.1051/medsci/2018309. Epub 2019 Jan 23. Med Sci (Paris). 2019. PMID: 30672459 Review. French. - VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours.
Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. Song E, et al. Nature. 2020 Jan;577(7792):689-694. doi: 10.1038/s41586-019-1912-x. Epub 2020 Jan 15. Nature. 2020. PMID: 31942068 Free PMC article. - How Do Meningeal Lymphatic Vessels Drain the CNS?
Raper D, Louveau A, Kipnis J. Raper D, et al. Trends Neurosci. 2016 Sep;39(9):581-586. doi: 10.1016/j.tins.2016.07.001. Epub 2016 Jul 25. Trends Neurosci. 2016. PMID: 27460561 Free PMC article. Review. - Meningeal Lymphatics: From Anatomy to Central Nervous System Immune Surveillance.
Papadopoulos Z, Herz J, Kipnis J. Papadopoulos Z, et al. J Immunol. 2020 Jan 15;204(2):286-293. doi: 10.4049/jimmunol.1900838. J Immunol. 2020. PMID: 31907271 Free PMC article. Review. - Meningeal lymphatic vessels regulate brain tumor drainage and immunity.
Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, He T, Ning W, Kong Y, Huo Y, He A, Liu B, Zhang J, Adams R, He Y, Tang F, Bian X, Luo J. Hu X, et al. Cell Res. 2020 Mar;30(3):229-243. doi: 10.1038/s41422-020-0287-8. Epub 2020 Feb 24. Cell Res. 2020. PMID: 32094452 Free PMC article.
Cited by
- The Predictive Value of Monocytes in Immune Microenvironment and Prognosis of Glioma Patients Based on Machine Learning.
Zhang N, Dai Z, Wu W, Wang Z, Cao H, Zhang Y, Wang Z, Zhang H, Cheng Q. Zhang N, et al. Front Immunol. 2021 Apr 16;12:656541. doi: 10.3389/fimmu.2021.656541. eCollection 2021. Front Immunol. 2021. PMID: 33959130 Free PMC article. - Machine learning revealed stemness features and a novel stemness-based classification with appealing implications in discriminating the prognosis, immunotherapy and temozolomide responses of 906 glioblastoma patients.
Wang Z, Wang Y, Yang T, Xing H, Wang Y, Gao L, Guo X, Xing B, Wang Y, Ma W. Wang Z, et al. Brief Bioinform. 2021 Sep 2;22(5):bbab032. doi: 10.1093/bib/bbab032. Brief Bioinform. 2021. PMID: 33839757 Free PMC article. - Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: The Role of the Complement and Innate Immune System.
Provencio JJ, Inkelas S, Vergouwen MDI. Provencio JJ, et al. Transl Stroke Res. 2024 Aug 21. doi: 10.1007/s12975-024-01290-5. Online ahead of print. Transl Stroke Res. 2024. PMID: 39168941 Review. - Immune and Clinical Features of CD96 Expression in Glioma by in silico Analysis.
Zhang Q, Zhong H, Fan Y, Liu Q, Song J, Yao S, Cao F. Zhang Q, et al. Front Bioeng Biotechnol. 2020 Jun 30;8:592. doi: 10.3389/fbioe.2020.00592. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32695752 Free PMC article. - Unravelling mysteries at the perivascular space: a new rationale for cerebral malaria pathogenesis.
Wassmer SC, de Koning-Ward TF, Grau GER, Pai S. Wassmer SC, et al. Trends Parasitol. 2024 Jan;40(1):28-44. doi: 10.1016/j.pt.2023.11.005. Epub 2023 Dec 8. Trends Parasitol. 2024. PMID: 38065791 Free PMC article. Review.
References
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. - PubMed
- Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–218. - PubMed
- Goldmann J, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. - PubMed
- Kaminski M, et al. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leukoc Biol. 2012;92:31–39. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 AI007496/AI/NIAID NIH HHS/United States
- R01 NS061973/NS/NINDS NIH HHS/United States
- R01 AG034113/AG/NIA NIH HHS/United States
- P30 CA044579/CA/NCI NIH HHS/United States
- R01AG034113/AG/NIA NIH HHS/United States
- R01NS061973/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous