Neurogenesis in the adult avian song-control system - PubMed (original) (raw)
Review
Neurogenesis in the adult avian song-control system
Eliot A Brenowitz et al. Cold Spring Harb Perspect Biol. 2015.
Abstract
New neurons are added throughout the forebrain of adult birds. The song-control system is a model to investigate the addition of new long-projection neurons to a cortical circuit that regulates song, a learned sensorimotor behavior. Neuroblasts destined for the song nucleus HVC arise in the walls of the lateral ventricle, and wander through the pallium to reach HVC. The survival of new HVC neurons is supported by gonadally secreted testosterone and its downstream effectors including neurotrophins, vascularization, and electrical activity of postsynaptic neurons in nucleus RA (robust nucleus of the arcopallium). In seasonal species, the HVC→RA circuit degenerates in nonbreeding birds, and is reconstructed by the incorporation of new projection neurons in breeding birds. There is a functional linkage between the death of mature HVC neurons and the birth of new neurons. Various hypotheses for the function of adult neurogenesis in the song system can be proposed, but this remains an open question.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
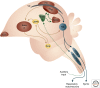
Figure 1.
A schematic of the neurogenic regions in the avian brain overlaid on the avian song circuits. Neurogenic regions are shown in red. Note the proximity of HVC (and hippocampus [HC]) to the ventricular zone (VZ). A schematic version of the motor pathway for song production is shown in blue. A schematic of the ascending auditory pathway is shown in green. The dotted line indicates an indirect route through many nuclei of the ascending auditory pathway leading to field L in the telencephalon. The anterior forebrain circuit for song learning and plasticity is shown in yellow. NCM, Caudomedial nidopallium; RA, arcopallium; LMAN, lateral magnocellular nucleus of the anterior neostriatum; OB, olfactory bulb; DLM, dorsolateral medial; PAm, parambigualis; RAm, retroambigualis
Figure 2.
A timeline of the processes of avian neurogenesis and the proportion of new neurons that persist through each of these processes. The timeline is based on the work of Alvarez-Buylla and Nottebohm (1988), Barami et al. (1994), and Scott et al. (2012). The percentages of survival through the various processes of avian neurogenesis are based on the findings of Kirn et al. (1991, 1999), Barnea and Nottebohm (1994), Nottebohm et al. (1994), Scott and Lois (2007), and Walton et al. (2012). VZ, Ventricular zone.
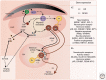
Figure 3.
A schematic of the factors ranging from gene expression to behavior that influence the birth and migration of neuroblasts and the addition of new neurons to an HVC. The solid black lines indicate factors that positively influence the given cell, factor, or process to which it points. The dashed lines represent changes in cell process indicated (e.g., cell divisions and migration). The dotted lines represent indirect routes of influence (i.e., through other genes, factors, etc.). The breakout panel to the right summarizes patterns of gene expression in different brain regions that positively influence component processes of neurogenesis. In the ventricular zone (VZ), the (+) indicates genes that promote proliferation, whereas the (−) indicates genes the promote exit from the cell cycle. DCX+, Doublecortin-positive migratory neuroblast; IN, interneuron; NPC, neural progenitor cell; NSC, neural stem cell; PN, projection neuron. ER, endoplasmic reticulum; VEGF, vascular endothelial growth factor; BDNF, brain-derived neurotrophic factor; GABA, γ-aminobutyric acid; RA, arcopallium; PAm, parambigualis; RAm, retroambigualis.
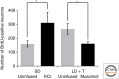
Figure 4.
Postsynaptic activity influences the addition of new robust nucleus of the arcopallium (RA)-projecting neurons to HVC. Spontaneous activity of neurons in RA was decreased by unilateral infusions of muscimol (2.8 mg/ml) or increased by infusion of KCl (100 m
m
). Inhibiting activity decreased neuronal addition (Larson et al. 2014). Increasing activity increased neuronal addition to HVC (ipsilateral to KCl infusion, 312 ± 75 new neurons; contralateral to KCl infusion, 160 ± 23 new neurons). Asterisks indicate p < 0.05 in post hoc _t_-test following two-way ANOVA. BrdU, 5-bromo-2′-deoxyuridine; SD, short day; LD, long day; T, testosterone.
Figure 5.
A model of the seasonal changes in physiology, morphology, and behavior of songbirds. As day length increases at the onset of the breeding season, plasma testosterone (T) levels increase. The increase in T drives an increase in neuronal number in HVC along with changes in gene expression, morphology, and physiology of HVC and robust nucleus of the arcopallium (RA). All of these changes in morphology and physiology permit the production of stereotyped song. As day length decreases at the onset of the nonbreeding season, T levels drop, HVC neurons die, and song degrades. This cycle repeats annually.
Similar articles
- Adult neurogenesis is necessary for functional regeneration of a forebrain neural circuit.
Brenowitz EA, Lent KL, Miller KE, Perkel DJ. Brenowitz EA, et al. Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2400596121. doi: 10.1073/pnas.2400596121. Epub 2024 Jul 5. Proc Natl Acad Sci U S A. 2024. PMID: 38968119 Free PMC article. - Adult Neurogenesis Leads to the Functional Reconstruction of a Telencephalic Neural Circuit.
Cohen RE, Macedo-Lima M, Miller KE, Brenowitz EA. Cohen RE, et al. J Neurosci. 2016 Aug 24;36(34):8947-56. doi: 10.1523/JNEUROSCI.0553-16.2016. J Neurosci. 2016. PMID: 27559175 Free PMC article. - Birth, migration, incorporation, and death of vocal control neurons in adult songbirds.
Alvarez-Buylla A, Kirn JR. Alvarez-Buylla A, et al. J Neurobiol. 1997 Nov;33(5):585-601. J Neurobiol. 1997. PMID: 9369461 Review. - Postsynaptic neural activity regulates neuronal addition in the adult avian song control system.
Larson TA, Wang TW, Gale SD, Miller KE, Thatra NM, Caras ML, Perkel DJ, Brenowitz EA. Larson TA, et al. Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16640-4. doi: 10.1073/pnas.1310237110. Epub 2013 Sep 23. Proc Natl Acad Sci U S A. 2013. PMID: 24062453 Free PMC article. - Testosterone and brain-derived neurotrophic factor interactions in the avian song control system.
Brenowitz EA. Brenowitz EA. Neuroscience. 2013 Jun 3;239:115-23. doi: 10.1016/j.neuroscience.2012.09.023. Epub 2012 Oct 30. Neuroscience. 2013. PMID: 23123886 Free PMC article. Review.
Cited by
- Regional Patterning of Adult Neurogenesis in the Homing Pigeon's Brain.
Mehlhorn J, Niski N, Liu K, Caspers S, Amunts K, Herold C. Mehlhorn J, et al. Front Psychol. 2022 Jul 8;13:889001. doi: 10.3389/fpsyg.2022.889001. eCollection 2022. Front Psychol. 2022. PMID: 35898980 Free PMC article. - Adult neurogenesis is necessary for functional regeneration of a forebrain neural circuit.
Brenowitz EA, Lent KL, Miller KE, Perkel DJ. Brenowitz EA, et al. Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2400596121. doi: 10.1073/pnas.2400596121. Epub 2024 Jul 5. Proc Natl Acad Sci U S A. 2024. PMID: 38968119 Free PMC article. - Predation drives the evolution of brain cell proliferation and brain allometry in male Trinidadian killifish, Rivulus hartii.
Dunlap KD, Corbo JH, Vergara MM, Beston SM, Walsh MR. Dunlap KD, et al. Proc Biol Sci. 2019 Dec 18;286(1917):20191485. doi: 10.1098/rspb.2019.1485. Epub 2019 Dec 11. Proc Biol Sci. 2019. PMID: 31822257 Free PMC article. - Plasticity in the hippocampal formation of shorebirds during the wintering period: Stereological analysis of parvalbumin neurons in Actitis macularius.
Guerreiro LCF, Henrique EP, da Silva Rosa JB, Pereira PDC, de Abreu CC, Fernandes TN, de Morais Magalhães NG, de Jesus Falcão da Silva A, da Costa ER, Guerreiro-Diniz C, Diniz CWP, Diniz DG. Guerreiro LCF, et al. Learn Behav. 2022 Mar;50(1):45-54. doi: 10.3758/s13420-021-00473-6. Epub 2021 Jul 9. Learn Behav. 2022. PMID: 34244975 - Exploring the Relationship between Brain Plasticity, Migratory Lifestyle, and Social Structure in Birds.
Barkan S, Yom-Tov Y, Barnea A. Barkan S, et al. Front Neurosci. 2017 Mar 27;11:139. doi: 10.3389/fnins.2017.00139. eCollection 2017. Front Neurosci. 2017. PMID: 28396621 Free PMC article.
References
- Adar E, Lotem A, Barnea A. 2008b. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res 187: 178–184. - PubMed
- Altman J, Das GD. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources