Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease - PubMed (original) (raw)
Review
Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease
Kou Takahashi et al. Cell Mol Life Sci. 2015 Sep.
Abstract
Glutamate is the predominant excitatory neurotransmitter in the central nervous system. Excitatory amino acid transporter 2 (EAAT2) is primarily responsible for clearance of extracellular glutamate to prevent neuronal excitotoxicity and hyperexcitability. EAAT2 plays a critical role in regulation of synaptic activity and plasticity. In addition, EAAT2 has been implicated in the pathogenesis of many central nervous system disorders. In this review, we summarize current understanding of EAAT2, including structure, pharmacology, physiology, and functions, as well as disease relevancy, such as in stroke, Parkinson's disease, epilepsy, amyotrophic lateral sclerosis, Alzheimer's disease, major depressive disorder, and addiction. A large number of studies have demonstrated that up-regulation of EAAT2 protein provides significant beneficial effects in many disease models suggesting EAAT2 activation is a promising therapeutic approach. Several EAAT2 activators have been identified. Further understanding of EAAT2 regulatory mechanisms could improve development of drug-like compounds that spatiotemporally regulate EAAT2.
Figures
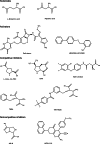
Fig. 1
Structure of substrate, activators, and inhibitors
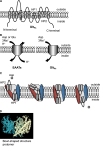
Fig. 2
Transport mechanism and structure of glutamate transporter. There are eight transmembrane (TM) domains and two helical hairpins (HP1 and HP2). a The topology model of an archaeal homolog of the EAATs from pyrococcus horikoshii, GltPh, is shown. TM 1, 2, 4, and 5 are included in the scaffold domain (trimerization domain) while TM 3, 6, 7, and 8, and HP1-2 are included in the core transport domain. b The stoichiometry of transport. EAATs exhibit influx of glutamate/aspartate (Glu), 3 Na+ and 1 H+, and outflux of 1 K+. GltPh utilizes influx of aspartate (Asp) and 3 Na+. c The hypothetical transport mechanism of GltPh. Reyes et al. proposed that the tips HP1 and HP2 contribute to substrate binding and transport [85] and are accompanied with rotation of the core transport domain (red). The trimerization domain is indicated in blue. d The bowl-shaped structure of the GltPh trimer. Each subunit is represented as blue, green, and white. The crystal structure is based on GltPh binding with TBOA (PBD 2NWW,
http://www.rcsb.org/pdb/home/home.do
)
Similar articles
- Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases.
Lin CL, Kong Q, Cuny GD, Glicksman MA. Lin CL, et al. Future Med Chem. 2012 Sep;4(13):1689-700. doi: 10.4155/fmc.12.122. Future Med Chem. 2012. PMID: 22924507 Free PMC article. - Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization.
Foran E, Rosenblum L, Bogush A, Pasinelli P, Trotti D. Foran E, et al. Glia. 2014 Aug;62(8):1241-53. doi: 10.1002/glia.22677. Epub 2014 Apr 21. Glia. 2014. PMID: 24753081 Free PMC article. - GLT-1 transporter: an effective pharmacological target for various neurological disorders.
Soni N, Reddy BV, Kumar P. Soni N, et al. Pharmacol Biochem Behav. 2014 Dec;127:70-81. doi: 10.1016/j.pbb.2014.10.001. Epub 2014 Oct 13. Pharmacol Biochem Behav. 2014. PMID: 25312503 Review. - Current approaches to enhance glutamate transporter function and expression.
Fontana AC. Fontana AC. J Neurochem. 2015 Sep;134(6):982-1007. doi: 10.1111/jnc.13200. Epub 2015 Aug 14. J Neurochem. 2015. PMID: 26096891 Review. - The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics.
Pajarillo E, Rizor A, Lee J, Aschner M, Lee E. Pajarillo E, et al. Neuropharmacology. 2019 Dec 15;161:107559. doi: 10.1016/j.neuropharm.2019.03.002. Epub 2019 Mar 6. Neuropharmacology. 2019. PMID: 30851309 Free PMC article. Review.
Cited by
- Inhibitory Potential of the Truncated Isoforms on Glutamate Transporter Oligomerization Identified by Computational Analysis of Gene-Centric Isoform Maps.
Karagöl A, Karagöl T, Li M, Zhang S. Karagöl A, et al. Pharm Res. 2024 Nov 1. doi: 10.1007/s11095-024-03786-z. Online ahead of print. Pharm Res. 2024. PMID: 39487385 - Astrocytic neuroligin 3 regulates social memory and synaptic plasticity through adenosine signaling in male mice.
Dang R, Liu A, Zhou Y, Li X, Wu M, Cao K, Meng Y, Zhang H, Gan G, Xie W, Jia Z. Dang R, et al. Nat Commun. 2024 Oct 5;15(1):8639. doi: 10.1038/s41467-024-52974-3. Nat Commun. 2024. PMID: 39366972 Free PMC article. - Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort.
Tourtourikov I, Todorov T, Angelov T, Chamova T, Tournev I, Mitev V, Todorova A. Tourtourikov I, et al. Genes (Basel). 2024 Sep 12;15(9):1197. doi: 10.3390/genes15091197. Genes (Basel). 2024. PMID: 39336788 Free PMC article. - Aberrant glycosylation in schizophrenia: insights into pathophysiological mechanisms and therapeutic potentials.
Feng Y, Sun L, Dang X, Liu D, Liao Z, Yao J, Zhang Y, Deng Z, Li J, Zhao M, Liu F. Feng Y, et al. Front Pharmacol. 2024 Sep 2;15:1457811. doi: 10.3389/fphar.2024.1457811. eCollection 2024. Front Pharmacol. 2024. PMID: 39286629 Free PMC article. Review. - Calcineurin inhibition prevents synaptic plasticity deficit induced by brain-derived tau oligomers.
Scaduto P, Marcatti M, Bhatt N, Kayed R, Taglialatela G. Scaduto P, et al. Brain Commun. 2024 Aug 16;6(5):fcae277. doi: 10.1093/braincomms/fcae277. eCollection 2024. Brain Commun. 2024. PMID: 39239152 Free PMC article.
References
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. - PubMed
- Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93:1621–1657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS064275/NS/NINDS NIH HHS/United States
- U01 NS074601/NS/NINDS NIH HHS/United States
- R01NS064275/NS/NINDS NIH HHS/United States
- U01NS074601/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical