Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing - PubMed (original) (raw)
Adaptive mutation in influenza A virus non-structural gene is linked to host switching and induces a novel protein by alternative splicing
Mohammed Selman et al. Emerg Microbes Infect. 2012 Nov.
Abstract
Little is known about the processes that enable influenza A viruses to jump into new host species. Here we show that the non-structural protein1 nucleotide substitution, A374G, encoding the D125G(GAT→GGT) mutation, which evolved during the adaptation of a human virus within a mouse host, activates a novel donor splice site in the non-structural gene, hence producing a novel influenza A viral protein, NS3. Using synonymous 125G mutations that do not activate the novel donor splice site, NS3 was shown to provide replicative gain-of-function. The protein sequence of NS3 is similar to NS1 protein but with an internal deletion of a motif comprised of three antiparallel β-strands spanning codons 126 to 168 in NS1. The NS1-125G(GGT) codon was also found in 33 natural influenza A viruses that were strongly associated with switching from avian to mammalian hosts, including human, swine and canine populations. In addition to the experimental human to mouse switch, the NS1-125G(GGT) codon was selected on avian to human transmission of the 1997 H5N1 and 1999 H9N2 lineages, as well as the avian to swine jump of 1979 H1N1 Eurasian swine influenza viruses, linking the NS1 125G(GGT) codon with host adaptation and switching among multiple species.
Keywords: NS gene; NS1; NS3; host switch; influenza A virus; non-structural protein 1; viral splicing.
Figures
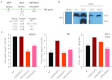
Figure 1
Host specific effects on viral replication. Virus growth in (A) MDCK , (B) A549, (C) M1cells (MOI 0.001, _n_=3) and (D) CD-1 mice (_n_=12) infected with influenza A viruses containing either the wt, M124I, D125G or the double-mutant (M124I+D125G) NS1 genes. (*P<0.05;**P<0.01; ***P<0.001; P value was determined by two-tailed student t with equal variance, comparing the mutants to wt 72 hpi or 1 day post-infection. Error bars indicate s.e.m.)
Figure 2
Presence of 20 kDa (NS3) band. (A) Immunobloting for endogenous NS1 in A549 or M1 cells infected with the wt, M124I, D125G or the double-mutant (M124I+D125G) NS1 gene viruses (MOI 3) or mock infected with PBS, 8 hpi. (B) Immunoblots for endogenous NS1 in M1 cells infected with a mouse adapted A/HK/1/68-11-MA21-1 virus possessing the NS1 D125G(GAT→GGT) mutation (MOI 0.5), human wt NS1 virus (MOI 3) or mock infected with PBS, 8 hpi. (C) Effect of NS mutations on the rate of viral protein synthesis in infected A459 cells. A549 cells were infected (MOI 5) and pulsed for 1 h with S35 8 hpi. Cell lysate was collected and used for SDS–PAGE and autoradiography.
Figure 3
Characterization of NS3 by mass spectrometry. (A) Workflow for the production and purification of NS1 and 20 kDa (NS3) proteins for LC–MS–MS sequencing. (B) Identification, relative abundance, and sequence of extracted ion chromatogram peaks of NS1 wt or mutant tryptic peptides found in the NS1 and NS3 protein. (C) Relative abundance of extracted ion chromatogram peaks of a joining tryptic peptide with NS1 amino acids 125 bonded to 168. The MS/MS spectrum for fragment ions of NS3 joining peptide is included in Supplementary Figure S2. Approximate position of tryptic peptide relative to NS1 and NS3 is shown on schematic maps of the NS1 and NS3 proteins. Sequence coverage is included in Supplementary Figure S1.
Figure 4
The NS1 D125G(GAT→GGT) mutation results in the formation of a novel splice product encoding NS3. (A) NS gene vRNA and mRNA from wt, M124I, D125G or the double-mutant (M124I+D125G) NS1 gene containing viruses were extracted from infected M1 cells (MOI 3) and amplified as cDNA by RT-PCR and were separated by agarose gel electrophoresis. Asterisk denotes bands corresponding to a NS1–NS3 hybrid artefact. (B) Schematic representations of NS transcripts, (NS1, NS3 and NEP), identified by sequencing of the PCR cDNA products. (C) Location of predicted alternative splice sites of wt, M124I, D125G and double-mutant (M124I+D125G) NS genes. (D) Nucleotide sequences of predicted splice site with likelihood scores (for sites in panel C) and resulting splice products (NEP or NS3) is shown for the NS1 wt, D125G and double-mutant (M124I+D125G). Human donor and acceptor splice site consensus sequences are shown above the splice sites of the NS genes. Position of the 124I and 125G mutations are highlighted in yellow and green respectively. (E–G), Levels of mRNA and vRNA. Quantitative RT-PCR was performed on total RNA from M1 cells infected with NS1 wt or mutant viruses (MOI 2), (_n_=3). (E) mRNA levels of NP, M1, NS1, NEP genes are shown relative to NS1-wt. (F) mRNA level of the NS3 transcripts. ND, Not detected. (E) vRNA levels of the NP, M, NS gene. (E, G) Results were normalized to β-actin levels, and presented as values relative to wt RNA levels. (E) Results were normalized as values relative to β-actin levels. (*P<0.05; **P<0.01; ***P<0.001; P value was determined by two-tailed student t with equal variance, (E,G) comparing the mutants to wt, (F) comparing the 125G to the double-mutant. Error bars indicate s.e.m.)
Figure 5
Synthetic production of NS3. (A) In vitro expression of wt and 125G(GGT) NS1 and NS3 from coupled T7 transcription and reticulocyte translation of expression plasmids or empty vector in the presence of 35S labeled methionine and cysteine. Cell lysate was collected and used for SDS–PAGE and autoradiography. (B) Immunoblots for NS1 in transfected 293T cells with NS1 (wt, 125G(GGT) or 125G(GGC) mutants) or NS3 expression plasmids. Cell lysate was collected 24 h post-transfection. Control immunoblot is shown for endogenous NS1 and NS3 proteins in M1 cells infected with mutant NS1.
Figure 6
Effect of novel NS splicing on viral replication. (A) Schematic representations of the NS gene and its corresponding splice sites with predicted proteins from the NS1-wt, and NS3 splicing competent 125G(GGT), and splicing incompetent 125G(GGC) and 125G(GGG) mutants. (B) Immunobloting for endogenous NS1 and NS3 protein production in M1 cells infected with HK NS1 125G(GGT), 125G(GGC) and 125G(GGG) viruses or mock infected with PBS, 24 hpi showing an absence of NS3 protein for viruses without the NS3 donor splice site. (C) Virus growth in MDCK (MOI 0.001; _n_=3), M1cells (MOI 0.001; _n_=3) and in CD-1 mice (_n_=3) infected with influenza A viruses containing the either NS1-wt, NS3 splicing competent NS1 125G(GGT), or splicing incompetent 125G(GGC) and 125G(GGG) mutants. (**P<0.01; ***P<0.001; P value was determined by two-tailed student t with equal variance, comparing the mutants or wt to the 125G(GGT) at 48 hpi or 1 day post-infection. Error bars indicate s.e.m.)
Similar articles
- An A14U Substitution in the 3' Noncoding Region of the M Segment of Viral RNA Supports Replication of Influenza Virus with an NS1 Deletion by Modulating Alternative Splicing of M Segment mRNAs.
Zheng M, Wang P, Song W, Lau SY, Liu S, Huang X, Mok BW, Liu YC, Chen Y, Yuen KY, Chen H. Zheng M, et al. J Virol. 2015 Oct;89(20):10273-85. doi: 10.1128/JVI.00919-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223635 Free PMC article. - Development of a dual-protective live attenuated vaccine against H5N1 and H9N2 avian influenza viruses by modifying the NS1 gene.
Choi EH, Song MS, Park SJ, Pascua PN, Baek YH, Kwon HI, Kim EH, Kim S, Jang HK, Poo H, Kim CJ, Choi YK. Choi EH, et al. Arch Virol. 2015 Jul;160(7):1729-40. doi: 10.1007/s00705-015-2442-y. Epub 2015 May 12. Arch Virol. 2015. PMID: 25959557 - Origins and Evolutionary Dynamics of H3N2 Canine Influenza Virus.
Zhu H, Hughes J, Murcia PR. Zhu H, et al. J Virol. 2015 May;89(10):5406-18. doi: 10.1128/JVI.03395-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740996 Free PMC article. - Mammalian adaptive mutations of the PA protein of highly pathogenic avian H5N1 influenza virus.
Yamaji R, Yamada S, Le MQ, Ito M, Sakai-Tagawa Y, Kawaoka Y. Yamaji R, et al. J Virol. 2015 Apr;89(8):4117-25. doi: 10.1128/JVI.03532-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631084 Free PMC article. - Isolation and genetic characterization of avian-like H1N1 and novel ressortant H1N2 influenza viruses from pigs in China.
Yu H, Zhang PC, Zhou YJ, Li GX, Pan J, Yan LP, Shi XX, Liu HL, Tong GZ. Yu H, et al. Biochem Biophys Res Commun. 2009 Aug 21;386(2):278-83. doi: 10.1016/j.bbrc.2009.05.056. Epub 2009 May 19. Biochem Biophys Res Commun. 2009. PMID: 19460353 Review.
Cited by
- Influenza A/Hong Kong/156/1997(H5N1) virus NS1 gene mutations F103L and M106I both increase IFN antagonism, virulence and cytoplasmic localization but differ in binding to RIG-I and CPSF30.
Dankar SK, Miranda E, Forbes NE, Pelchat M, Tavassoli A, Selman M, Ping J, Jia J, Brown EG. Dankar SK, et al. Virol J. 2013 Jul 25;10:243. doi: 10.1186/1743-422X-10-243. Virol J. 2013. PMID: 23886034 Free PMC article. - Crucial role of PA in virus life cycle and host adaptation of influenza A virus.
Hu J, Liu X. Hu J, et al. Med Microbiol Immunol. 2015 Apr;204(2):137-49. doi: 10.1007/s00430-014-0349-y. Epub 2014 Jul 29. Med Microbiol Immunol. 2015. PMID: 25070354 Review. - Analysis of Single Nucleotide Variants (SNVs) Induced by Passages of Equine Influenza Virus H3N8 in Embryonated Chicken Eggs.
Rozek W, Kwasnik M, Socha W, Sztromwasser P, Rola J. Rozek W, et al. Viruses. 2021 Aug 5;13(8):1551. doi: 10.3390/v13081551. Viruses. 2021. PMID: 34452416 Free PMC article. - PA-X is an avian virulence factor in H9N2 avian influenza virus.
Clements AL, Peacock TP, Sealy JE, Lee HM, Hussain S, Sadeyen JR, Shelton H, Digard P, Iqbal M. Clements AL, et al. J Gen Virol. 2021 Mar;102(3):001531. doi: 10.1099/jgv.0.001531. Epub 2021 Feb 5. J Gen Virol. 2021. PMID: 33544070 Free PMC article. - Roles and functions of IAV proteins in host immune evasion.
Rashid F, Xie Z, Li M, Xie Z, Luo S, Xie L. Rashid F, et al. Front Immunol. 2023 Dec 13;14:1323560. doi: 10.3389/fimmu.2023.1323560. eCollection 2023. Front Immunol. 2023. PMID: 38152399 Free PMC article. Review.
References
- Fields BN, Knipe DM, Howley PM. Fields virology. 5th ed. Philadelphia; Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
- Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous