Astrocytes Are Primed by Chronic Neurodegeneration to Produce Exaggerated Chemokine and Cell Infiltration Responses to Acute Stimulation with the Cytokines IL-1β and TNF-α - PubMed (original) (raw)
Astrocytes Are Primed by Chronic Neurodegeneration to Produce Exaggerated Chemokine and Cell Infiltration Responses to Acute Stimulation with the Cytokines IL-1β and TNF-α
Edel Hennessy et al. J Neurosci. 2015.
Abstract
Microgliosis and astrogliosis are standard pathological features of neurodegenerative disease. Microglia are primed by chronic neurodegeneration such that toll-like receptor agonists, such as LPS, drive exaggerated cytokine responses on this background. However, sterile inflammatory insults are more common than direct CNS infection in the degenerating brain and these insults drive robust IL-1β and TNF-α responses. It is unclear whether these pro-inflammatory cytokines can directly induce exaggerated responses in the degenerating brain. We hypothesized that glial cells in the hippocampus of animals with chronic neurodegenerative disease (ME7 prion disease) would display exaggerated responses to central cytokine challenges. TNF-α or IL-1β were administered intrahippocampally to ME7-inoculated mice and normal brain homogenate-injected (NBH) controls. Both IL-1β and TNF-α produced much more robust IL-1β synthesis in ME7 than in NBH animals and this occurred exclusively in microglia. However, there was strong nuclear localization of the NFκB subunit p65 in the astrocyte population, associated with marked astrocytic synthesis of the chemokines CXCL1 and CCL2 in response to both cytokine challenges in ME7 animals. Conversely, very limited expression of these chemokines was apparent in NBH animals similarly challenged. Thus, astrocytes are primed in the degenerating brain to produce exaggerated chemokine responses to acute stimulation with pro-inflammatory cytokines. Furthermore, this results in markedly increased neutrophil, T-cell, and monocyte infiltration in the diseased brain. These data have significant implications for acute sterile inflammatory insults such as stroke and traumatic brain injury occurring on a background of aging or neurodegeneration.
Keywords: astrocyte; chemokine; cytokine; microglia; neurodegeneration; neuroinflammation.
Copyright © 2015 Hennessy et al.
Figures
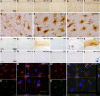
Figure 1.
Microglial priming, activation, and IL-1β synthesis. Microglial activation status was examined at 2 h post-intrahippocampal IL-1β (10 ng) or TNF-α (300 ng) challenge in NBH and ME7 animals. Hippocampal CA1 IBA-1 microglial labeling ×20 (a–f) and ×100 (g–i). Hippocampal TNF-α labeling ×5 (j–o). n, Inset shows TNF-α labeling, post-intrahippocampal LPS, with and without pre-absorption of the antibody with excess TNF-α. Hippocampal IL-1β labeling is shown at ×20 (p–u). u, Inset shows IL-1β-positive microglia adjacent to unlabeled astrocytic-like nucleus. Fluorescent double labeling of IBA-1 (594 nm) with IL-1β (488 nm) and Hoechst 33258 (blue) in ME7 animals 2 h post IL-1β (I–III) or TNF-α (IV–VI).
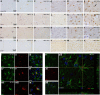
Figure 2.
Astrocyte priming, activation, and chemokine synthesis. NFκB activation and chemokine expression 2 h post intrahippocampal IL-1β (10 ng) or TNF-α (300 ng) challenge of NBH and ME7 animals. NFκB p65 labeling (×100) in the hippocampus (a–f) with astrocytic-like nucleus indicated by white arrow and microglial-like nucleus indicated by black arrow in f. Hippocampal CCL2 labeling at ×100 magnification (g–l). CXCL1 labeling at ×100 magnification (m–r). l, r, Insets show CCL2 and CXCL1 labeling, respectively, associated with astrocyte-like nucleus adjacent to microglia-like nucleus devoid of labeling. Hippocampal CA1 GFAP astrocyte labeling in NBH and ME7 animals is shown at ×20 (s–x). Fluorescent labeling of GFAP (488 nm), p65 (633 nm), CCL2 (594 nm), and CXCL1 (594 nm) 2 h post TNF-α (i–ix) or IL-1β challenge (x–xiii).
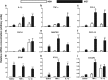
Figure 3.
Analysis of cytokine, chemokine, and astrocytosis transcripts. Hippocampal expression of transcripts for inflammatory genes was analyzed 2 h post intrahippocampal IL-1β (10 ng) or TNF-α (300 ng). a, IL-1β; b, TNF-α; c, CCL2; d, CXCL1; e, RANTES; f, CXCL10; g, GFAP; h, PTX3; i, STEAP4. Data were analyzed using two-way ANOVA and main effects and interactions are described in the main text. Statistically significant differences between NBH and ME7 by Bonferroni post hoc tests are denoted by ***p < 0.001, **p < 0.01, and *p < 0.05. All data are represented as the mean ± SEM and n = 4 for all groups except for the analysis of TNF-α, CCL2, and CXCL10, in which both ME7 + saline and ME7 + IL-1β had n = 6.
Figure 4.
Cytokine-induced neutrophil infiltration. Hippocampal neutrophil infiltration at 24 h post intrahippocampal IL-1β (10 ng) or TNF-α (300 ng) challenge. MBS-1 neutrophil labeling at ×20 magnification (a–f) at 24 h post challenge. Quantification of neutrophils in an area of 4.64 mm2 centered on the injected dorsal hippocampus following IL-1β (g) and TNF-α (h) injection. Data were analyzed using a two-way ANOVA followed by Bonferroni post hoc test. Interactions between treatment and disease are denoted by #p < 0.05 and ##p < 0.01. All data are represented as the mean ± SEM, n = 3–5 for all groups.
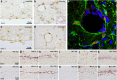
Figure 5.
Chemokine expression and immune cell recruitment at brain barriers. Chemokine expression at ventricular and vascular surfaces at 2 h post intrahippocampal cytokine challenge. Representative images of CCL2 at the ventricular (a) and vascular (b) surfaces of ME7 animals ×100 at 2 h. b, Inset shows CCL2 labeling with and without pre-absorption of the antibody with excess CCL2. CXCL-1 at the ventricular (c) and vascular (d) surfaces ×100 at 2 h. d, Inset shows CXCL1 labeling with and without pre-absorption of the antibody with excess CXCL1. GFAP and CCL2 colocalization at a hippocampal blood vessel at 2 h post TNF-α (e). MBS-1 neutrophil labeling at the ventricular membrane at 24 h post challenge with IL-1β (10 ng) or TNF-α (300 ng) challenge (×40, f–k). CD68 macrophage labeling (×40, l–q) at the ventricular surface (glia limitans) at 24 h post challenge with IL-1β (10 ng) or TNF-α (300 ng).
Figure 6.
Immune cell infiltration at 72 h. Cellular infiltration at 72 h post intrahippocampal TNF-α (300 ng) challenge. Hippocampal MBS-1 neutrophil labeling ×40 (a–d), CD3 T-cell labeling ×40 (e–h), and Pu.1 macrophage/microglial labeling ×20 (i–l). Quantification of MBS-1-positive neutrophils (m) and CD3-positive T-cells (n) in the injected hippocampus at 72 h. Pu.1-positive macrophages and microglia were quantified per 0.29 mm2 of the injected hippocampus (o). The latter were quantified in the presence and absence of peripherally administered Mc21 to deplete circulating monocytes. Data were analyzed using two-way ANOVA (full analysis in main text) followed by Bonferroni post hoc test. Selected statistically significant pairwise comparisons are denoted by *p < 0.05, **p < 0.01, ***p < 0.001. All data are represented as the mean ± SEM, n = 3–7.
Similar articles
- Systemic TNF-α produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration.
Hennessy E, Gormley S, Lopez-Rodriguez AB, Murray C, Murray C, Cunningham C. Hennessy E, et al. Brain Behav Immun. 2017 Jan;59:233-244. doi: 10.1016/j.bbi.2016.09.011. Epub 2016 Sep 12. Brain Behav Immun. 2017. PMID: 27633985 Free PMC article. - Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6.
Murray CL, Skelly DT, Cunningham C. Murray CL, et al. J Neuroinflammation. 2011 May 17;8:50. doi: 10.1186/1742-2094-8-50. J Neuroinflammation. 2011. PMID: 21586125 Free PMC article. - Acute systemic inflammation exacerbates neuroinflammation in Alzheimer's disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction.
Lopez-Rodriguez AB, Hennessy E, Murray CL, Nazmi A, Delaney HJ, Healy D, Fagan SG, Rooney M, Stewart E, Lewis A, de Barra N, Scarry P, Riggs-Miller L, Boche D, Cunningham MO, Cunningham C. Lopez-Rodriguez AB, et al. Alzheimers Dement. 2021 Oct;17(10):1735-1755. doi: 10.1002/alz.12341. Epub 2021 Jun 3. Alzheimers Dement. 2021. PMID: 34080771 Free PMC article. - Neuroinflammation, Microglia, and Cell-Association during Prion Disease.
Carroll JA, Chesebro B. Carroll JA, et al. Viruses. 2019 Jan 15;11(1):65. doi: 10.3390/v11010065. Viruses. 2019. PMID: 30650564 Free PMC article. Review.
Cited by
- Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage.
Guo Y, Dai W, Zheng Y, Qiao W, Chen W, Peng L, Zhou H, Zhao T, Liu H, Zheng F, Sun P. Guo Y, et al. Molecules. 2022 Oct 20;27(20):7080. doi: 10.3390/molecules27207080. Molecules. 2022. PMID: 36296682 Free PMC article. Review. - Neutrophils and macrophages drive TNF-induced lethality via TRIF/CD14-mediated responses.
Muendlein HI, Connolly WM, Cameron J, Jetton D, Magri Z, Smirnova I, Vannier E, Li X, Martinot AJ, Batorsky R, Poltorak A. Muendlein HI, et al. Sci Immunol. 2022 Dec 23;7(78):eadd0665. doi: 10.1126/sciimmunol.add0665. Epub 2022 Dec 23. Sci Immunol. 2022. PMID: 36563168 Free PMC article. - The Importance of CXCL1 in Physiology and Noncancerous Diseases of Bone, Bone Marrow, Muscle and the Nervous System.
Korbecki J, Gąssowska-Dobrowolska M, Wójcik J, Szatkowska I, Barczak K, Chlubek M, Baranowska-Bosiacka I. Korbecki J, et al. Int J Mol Sci. 2022 Apr 11;23(8):4205. doi: 10.3390/ijms23084205. Int J Mol Sci. 2022. PMID: 35457023 Free PMC article. Review. - Astrocytes: Heterogeneous and Dynamic Phenotypes in Neurodegeneration and Innate Immunity.
Cunningham C, Dunne A, Lopez-Rodriguez AB. Cunningham C, et al. Neuroscientist. 2019 Oct;25(5):455-474. doi: 10.1177/1073858418809941. Epub 2018 Nov 17. Neuroscientist. 2019. PMID: 30451065 Free PMC article. Review. - Unwanted Exacerbation of the Immune Response in Neurodegenerative Disease: A Time to Review the Impact.
Leite AOF, Bento Torres Neto J, Dos Reis RR, Sobral LL, de Souza ACP, Trévia N, de Oliveira RB, Lins NAA, Diniz DG, Diniz JAP, Vasconcelos PFDC, Anthony DC, Brites D, Picanço Diniz CW. Leite AOF, et al. Front Cell Neurosci. 2021 Oct 22;15:749595. doi: 10.3389/fncel.2021.749595. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34744633 Free PMC article. Review.
References
- Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–467. doi: 10.1002/glia.22616. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources