Nuclear pore complex integrity requires Lnp1, a regulator of cortical endoplasmic reticulum - PubMed (original) (raw)
Nuclear pore complex integrity requires Lnp1, a regulator of cortical endoplasmic reticulum
Amanda K Casey et al. Mol Biol Cell. 2015.
Abstract
The nuclear envelope (NE) and endoplasmic reticulum (ER) are components of the same contiguous membrane system and yet have distinct cellular functions. Mounting evidence suggests roles for some ER proteins in the NE for proper nuclear pore complex (NPC) structure and function. In this study, we identify a NE role in Saccharomyces cerevisiae for Lnp1 and Sey1, proteins required for proper cortical ER formation. Both lnp1Δ and sey1Δ mutants exhibit synthetic genetic interactions with mutants in genes encoding key NPC structural components. Both Lnp1 and Sey1 physically associate with other ER components that have established NPC roles, including Rtn1, Yop1, Pom33, and Per33. Of interest, lnp1Δ rtn1Δ mutants but not rtn1Δ sey1Δ mutants exhibit defects in NPC distribution. Furthermore, the essential NPC assembly factor Ndc1 has altered interactions in the absence of Sey1. Lnp1 dimerizes in vitro via its C-terminal zinc finger motif, a property that is required for proper ER structure but not NPC integrity. These findings suggest that Lnp1's role in NPC integrity is separable from functions in the ER and is linked to Ndc1 and Rtn1 interactions.
© 2015 Casey et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
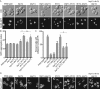
FIGURE 1:
lnp1Δ rtn1Δ cells have defects in NPC organization. (A) Parental or mutant cells expressing Nic96-GFP were grown to early log phase at 25°C and visualized by fluorescence microscopy. Scale bar, 5 μm. (B) The aggregation indexes of Nic96-GFP–expressing cells. Error bars represent SE. Asterisk denotes statistical significance (p < 0.01). n.s., no statistical significance. (C) Percentages of cells with regions of collapsed cortical ER as indicated by lack of peripheral Sec61-GFP staining as quantified from images of Sec61-GFP–expressing cells. Error bars represent SE. Asterisk denotes statistical significance (p < 0.01). (D) Parental or mutant cells expressing Sec61-GFP grown to early log phase at 25°C and visualized by fluorescence microscopy. Scale bar, 5 μm. Arrows mark typical regions of collapsed cortical ER in represented cells. Arrowheads for lnp1Δ rtn1Δ highlight aberrant folds in cortical ER.
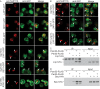
FIGURE 2:
Lnp1 and Sey1 localize to the NE and physically interact with shared ER and NPC components. (A, B) Indirect immunofluorescence microscopy was performed with cells using chicken anti-GFP and rabbit anti-Nup116C antibodies. Arrows indicate NPC clusters. (C) Yeast lysates were prepared from cells expressing Pom33-FLAG, Per33-FLAG, Lnp1-HA, Pom33-FLAG and Lnp1-HA, or Per33-FLAG and Lnp1-HA. Lysates were immunoprecipitated with anti-FLAG affinity matrix and blotted using anti-HA antibodies. Asterisk indicates contaminant band. (D) Yeast lysates were prepared from cells expressing Pom33-FLAG, Per33-FLAG, Sey1-HA, Pom33-FLAG and Sey1-HA, or Per33-FLAG and Sey1-HA. Lysates were immunoprecipitated with anti-FLAG affinity matrix and blotted using anti-HA antibodies.
FIGURE 3:
lnp1Δ and sey1Δ mutants genetically interact with mutants in genes of the Nup84 subcomplex. (A) lnp1Δ pom33Δ and lnp1Δ rtn1Δ yop1Δ pom33Δ mutants have enhanced growth defects. Yeast strains were grown at 25°C and fivefold serially diluted onto plates of rich media incubated at the listed temperatures. (B) lnp1Δ nup133Δ and sey1Δ nup133Δ mutants have enhanced growth defects. Yeast strains were grown at 25°C and fivefold serially diluted onto plates of rich media incubated at the temperatures indicated.
FIGURE 4:
The function of Lnp1 and Sey1 with NPCs is coupled with the interaction between Rtn1 and the NPC. (A) Split ubiquitin yeast two-hybrid vectors containing genes encoding either NubG-Yop1 or NubG-Rtn1 (preys) were expressed in wild-type or mutant strains and tested for interaction with Ndc1-Cub (Bait). Presence of both bait and prey plasmids was detected on SCM-Leu-Trp. Interaction of bait and prey was assayed by growth on SCM-Leu-Trp-His-Ade at 25°C with and without 3-AT. (B) Yeast lysates were prepared from cells expressing Ndc1-TAP, Rtn1-GFP, and Ndc1-TAP and Rtn1-GFP in wild-type or sey1Δ mutant strains. Lysates were immunoprecipitated with GFP-binding protein resin and blotted using anti-GFP and anti-mouse IgG antibodies. (C) lnp1Δ nup133Δ, nup133Δ lnp1Δ nup133Δ, and sey1Δ nup133Δ mutants were transformed with plasmids encoding RTN1, rtn1-K48I, or empty vector, grown to early log phase at 25°C, and fivefold serially diluted onto SCM-Leu plates at the indicated temperatures. (D) Cells expressing either Rtn1-GFP or rtn1-K48I-GFP were grown to early log phase at 25°C, induced for overexpression of Nup53-mcherry for 8 h, and visualized by fluorescence microscopy. Arrows indicate nuclear karmellae. Scale bar, 5 μm.
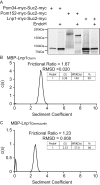
FIGURE 5:
The C-terminal zinc finger domain of Lnp1 is required for dimerization in vitro. (A) Lysates from cells expressing Pom34-myc-Suc2-myc, Pom152-myc-Suc2-myc, or Lnp1-myc-Suc2-myc were either mock digested or treated with EndoH and analyzed by immunoblotting with mouse anti-Myc antibody. (B, C) Sedimentation velocity analytical ultracentrifugation was performed with recombinant MBP-Lnp1Cterm and MBP-Lnp1CtermΔznfn. Determined molecular masses are given for major species.
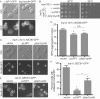
FIGURE 6:
The zinc finger of Lnp1 is not required for NPC function. (A) Parental cells exogenously expressing either Lnp1-GFP or lnp1Δ_znfn-GFP were grown to early log phase at 25°C and visualized by fluorescence microscopy. Scale bar, 5 μm. (B) Expression of lnp1Δznfn results in rescue of lnp1Δ nup133Δ. The lnp1Δ nup133Δ mutants were transformed with pLNP1, plnp1Δznfn, or empty vector and grown to early log phase at 25°C, fivefold serially diluted, and grown at indicated temperatures. (C) Expression of lnp1Δznfn results in rescue of lnp1Δ rtn1Δ NPC aggregation. lnp1Δ rtn1Δ NIC96-GFP mutants were transformed with pLNP1, plnp1Δznfn, or empty vector and grown to early log phase at 25°C and imaged. Scale bar, 5 μm. (D) The aggregation indexes of Nic96-GFP–expressing cells. Error bars represent SE. Asterisk denotes statistical significance (p < 0.01). (E) Expression of lnp1Δznfn is not sufficient to rescue lnp1Δ defects in ER. lnp1Δ SEC61-GFP mutants were transformed with pLNP1, p_lnp1Δznfn, or empty vector and grown to early log phase at 25°C and imaged. Scale bar, 5 μm. (F) Percentages of cells with regions of collapsed cortical ER as indicated by lack of peripheral Sec61-GFP staining were quantified from images of Sec61-GFP–expressing cells. Error bars represent SE. Asterisk denotes statistical significance (p < 0.01).
Similar articles
- Integrity and function of the Saccharomyces cerevisiae spindle pole body depends on connections between the membrane proteins Ndc1, Rtn1, and Yop1.
Casey AK, Dawson TR, Chen J, Friederichs JM, Jaspersen SL, Wente SR. Casey AK, et al. Genetics. 2012 Oct;192(2):441-55. doi: 10.1534/genetics.112.141465. Epub 2012 Jul 13. Genetics. 2012. PMID: 22798490 Free PMC article. - Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution.
Chadrin A, Hess B, San Roman M, Gatti X, Lombard B, Loew D, Barral Y, Palancade B, Doye V. Chadrin A, et al. J Cell Biol. 2010 May 31;189(5):795-811. doi: 10.1083/jcb.200910043. Epub 2010 May 24. J Cell Biol. 2010. PMID: 20498018 Free PMC article. - ER membrane-bending proteins are necessary for de novo nuclear pore formation.
Dawson TR, Lazarus MD, Hetzer MW, Wente SR. Dawson TR, et al. J Cell Biol. 2009 Mar 9;184(5):659-75. doi: 10.1083/jcb.200806174. J Cell Biol. 2009. PMID: 19273614 Free PMC article. - Nuclear envelope insertion of spindle pole bodies and nuclear pore complexes.
Jaspersen SL, Ghosh S. Jaspersen SL, et al. Nucleus. 2012 May-Jun;3(3):226-36. doi: 10.4161/nucl.20148. Epub 2012 May 1. Nucleus. 2012. PMID: 22572959 Free PMC article. Review. - Nuclear pore complex biogenesis.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2009 Aug;21(4):603-12. doi: 10.1016/j.ceb.2009.05.001. Epub 2009 Jun 11. Curr Opin Cell Biol. 2009. PMID: 19524430 Free PMC article. Review.
Cited by
- Regulated interaction of ID2 with the anaphase-promoting complex links progression through mitosis with reactivation of cell-type-specific transcription.
Lee SB, Garofano L, Ko A, D'Angelo F, Frangaj B, Sommer D, Gan Q, Kim K, Cardozo T, Iavarone A, Lasorella A. Lee SB, et al. Nat Commun. 2022 Apr 19;13(1):2089. doi: 10.1038/s41467-022-29502-2. Nat Commun. 2022. PMID: 35440621 Free PMC article. - Lunapark-dependent formation of a virus-induced ER exit site contains multi-tubular ER junctions that promote viral ER-to-cytosol escape.
Bagchi P, Liu X, Cho WJ, Tsai B. Bagchi P, et al. Cell Rep. 2021 Dec 7;37(10):110077. doi: 10.1016/j.celrep.2021.110077. Cell Rep. 2021. PMID: 34879280 Free PMC article. - Spindle Dynamics during Meiotic Development of the Fungus Podospora anserina Requires the Endoplasmic Reticulum-Shaping Protein RTN1.
López-Fuentes AJ, Nachón-Garduño KN, Suaste-Olmos F, Mendieta-Romero A, Peraza-Reyes L. López-Fuentes AJ, et al. mBio. 2021 Oct 26;12(5):e0161521. doi: 10.1128/mBio.01615-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607459 Free PMC article. - Quantitative proteomics reveal proteins enriched in tubular endoplasmic reticulum of Saccharomyces cerevisiae.
Wang X, Li S, Wang H, Shui W, Hu J. Wang X, et al. Elife. 2017 Mar 13;6:e23816. doi: 10.7554/eLife.23816. Elife. 2017. PMID: 28287394 Free PMC article. - Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing.
Webster BM, Thaller DJ, Jäger J, Ochmann SE, Borah S, Lusk CP. Webster BM, et al. EMBO J. 2016 Nov 15;35(22):2447-2467. doi: 10.15252/embj.201694574. Epub 2016 Oct 12. EMBO J. 2016. PMID: 27733427 Free PMC article.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
- Antonin W, Ellenberg J, Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM057438/GM/NIGMS NIH HHS/United States
- 1S10OD012324/OD/NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- 5R01 GM057438/GM/NIGMS NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- Howard Hughes Medical Institute/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 GM035370/GM/NIGMS NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- S10 OD012324/OD/NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases