Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response - PubMed (original) (raw)
Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response
Andrew E H Elia et al. Mol Cell. 2015.
Abstract
Execution of the DNA damage response (DDR) relies upon a dynamic array of protein modifications. Using quantitative proteomics, we have globally profiled ubiquitination, acetylation, and phosphorylation in response to UV and ionizing radiation. To improve acetylation site profiling, we developed the strategy FACET-IP. Our datasets of 33,500 ubiquitination and 16,740 acetylation sites provide valuable insight into DDR remodeling of the proteome. We find that K6- and K33-linked polyubiquitination undergo bulk increases in response to DNA damage, raising the possibility that these linkages are largely dedicated to DDR function. We also show that Cullin-RING ligases mediate 10% of DNA damage-induced ubiquitination events and that EXO1 is an SCF-Cyclin F substrate in the response to UV radiation. Our extensive datasets uncover additional regulated sites on known DDR players such as PCNA and identify previously unknown DDR targets such as CENPs, underscoring the broad impact of the DDR on cellular physiology.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
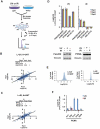
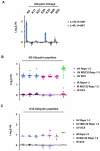
Figure 1. Quantitative profiling of ubiquitination in response to ultraviolet and ionizing radiation
(A) Schematic overview of approach to profile ubiquitination regulated by UV or IR. Three biological replicates were performed for each stimulus and 3 sequential IPs for each replicate, giving a total of 9 IPs per stimulus. (B, C) Experimental reproducibility indicated by Pearson correlation coefficients for Log2(L/H) ratios for 3 biological UV replicates (B) and for 3 biological IR replicates (C). (D) Log2(L/H) ratios for diGly sites on proteins known to be ubiquitinated in response to UV and/or IR. For both FANCD2 and PCNA, the reduced or absent levels of IR-induced ubiquitination, relative to UV-induced ubiquitination, are consistent between proteomic and immunoblot results. (E) Histograms depicting Log2(L/H) ratios for all quantified diGly sites in the 3 UV replicates (left) and 3 IR replicates (right). (F) Log2(L/H) ratios for all quantified sites of PCNA ubiquitination found in both the UV and IR datasets. Error bars in all plots represent the SEM of unique MS1 quantifications for the indicated site. See also Figure S1.
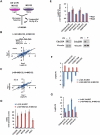
Figure 2. Profiling of UV- and IR-regulated ubiquitination in the presence of proteasome inhibition
(A) Schematic representation of DDR ubiquitin profiling in the presence of proteasome inhibition. MG132 was added to both damaged (light) and non-damaged (heavy) plates to capture ubiquitination events that lead to proteasomal degradation. (B, C) Pearson correlation coefficients for Log2(L/H) ratios for 3 biological UV replicates in the presence of MG132 (B) and for 2 biological IR replicates in the presence of MG132 (C). (D) MG132 increases detection of diGly peptides from proteins whose UV-induced ubiquitination is known to cause proteasomal degradation. Mean diGly TSCs (total spectral counts) depicted in blue are derived from the UV screen (Figure 1A), while mean diGly TSCs in red are from the UV-MG132 screen (Figure 2A). In the absence of MG132, diGly peptides from SETD8, CDC6, and EXO1 are not detected at all. (E) Quantitation of diGly sites on proteins whose UV-induced ubiquitination is known to result in proteasomal degradation. Log2(L/H) ratios come from the UV-MG132 screen in Figure 2A. (F) In the absence of MG132 (blue bars), diGly sites on degraded proteins decrease in response to UV stimulation, while pre-treatment with MG132 (red bars) reverses this decrease. Blue bars are from UV screens (Figures 1A or S4A) and red bars from the UV-MG132 screen (Figure 2A). (G) The UV induction of known non-degradative ubiquitination events is lower in the presence (red bars) than in the absence (blue bars) of MG132. Blue bars are from the UV dataset (Figure 1A) and red bars from the UV-MG132 dataset (Figure 2A). Error bars in all plots represent the SEM of unique MS1 quantifications for the indicated site. See also Figure S1.
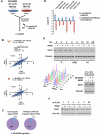
Figure 3. DDR-regulated ubiquitination of mitotic spindle proteins
(A) Biological pathways and functions significantly enriched among proteins ubiquitinated in response to UV radiation in the UV, UV-MG132, or UV-SCX datasets. Analysis was performed with Ingenuity and significance determined using the right-tailed Fisher's exact test. (B) Log2(L/H) ratios for diGly sites on centromere proteins (CENPs) deubiquitinated in response to UV and/or IR in the UV-SCX or IR-SCX datasets. (C) Mitotic spindle proteins ubiquitinated or deubiquitinated in response to UV (rectangles), IR (ovals), or both (rectangular oval). Green proteins (Log2 ratios >1) are ubiquitinated and red proteins (Log2 ratios <−1) deubiquitinated. Data are from the UV, UV-MG132, UV-SCX, IR, IR-MG, or IR-SCX datasets.
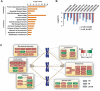
Figure 4. FACET-IP identifies acetylation sites regulated by ultraviolet or ionizing radiation
(A) Schematic for proteomic identification of acetylation sites by FACET-IP. (B) Number of acetylation sites (blue) and acetylated proteins (red) detected by FACET-IP among 10 SCX fractions (range 745-2556 sites, 523-1220 proteins). Elution of fractions 1-5 occurred using a KCl gradient to 91 mM and fractions 6-10 using isocratic 350 mM KCl. Dashed lines represent the number of acetylated sites (1006 sites, blue) and proteins (554 proteins, red) identified in a single IP from 20 mg of nuclear lysates. (C) Number of acetylated sites and proteins identified, quantified, and upregulated at least 2-fold in response to UV and IR. (D) Identification of site K382 on TP53 known to be acetylated in response to UV and IR. (E) Heat map representing Log2(L/H) ratios for ubiquitination (upper) and acetylation (lower) among 237 quantified sites that were both acetylated and ubiquitinated and that underwent a more than 2-fold increase in ubiquitination in response to UV. (F) Percentage of quantified ubiquitination, acetylation, and phosphorylation sites that are upregulated at least 2-fold in response to UV. All values are derived from proteomic screens (anti-diGly, anti-acK, or IMAC) involving SCX fractionation of nuclear lysates. (G) Heat map representing the number of UV or IR-inducible acetylation, ubiquitination, and phosphorylation sites on DNA repair proteins (belonging to the Gene Ontology category GO:000628) from six prior proteomic studies, as described in Experimental Procedures. See also Figures S2-S4.
Figure 5. K6- and K33-linked polyubiquitination occurs in response to UV radiation
(A) Log2(L/H) ratios for ubiquitin diGly sites from cells treated with UV (blue bars) or IR (red bars). Data are from the UV and IR datasets in Figure 1A. Error bars represent the SEM of multiple MS1 quantifications for the indicated site. (B) Log2(L/H) ratios for ubiquitin peptides modified on Lys-6 with diGly. For UV stimulation, all 76 unique peptide measurements across 7 biological replicates demonstrate upregulation, whereas none of 68 IR peptide measurements across 6 biological replicates are regulated. (C) Log2(L/H) ratios for ubiquitin peptides modified on Lys-33 with diGly. For UV stimulation, all 27 unique peptide measurements across 7 biological replicates demonstrate upregulation, whereas none of 21 IR peptide measurements across 6 biological replicates are regulated. Gray bars in (B) and (C) represent median and standard deviation of Log2(L/H) values.
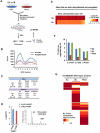
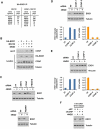
Figure 6. Screen for CRL-mediated ubiquitination events in the DDR identifies EXO1 as an SCF substrate
(A) Diagram of approach to identify UV or IR induced ubiquitination events mediated by CRLs. Light cells were treated with 10 ιM MLN4924 and 5 ιM MG132 for 30 minutes while heavy cells were treated with 5 ιM MG132 for 30 minutes. Both heavy and light plates were then irradiated with 40 J/m2 UV or 10 Gy IR and harvested 1 hour later. (B) Correlation coefficients for Log2(L/H) ratios for 3 biological replicates identifying CRL substrates in the presence of UV and for 2 biological replicates in the presence of IR. (C) Overlap of sites and proteins ubiquitinated more than 2-fold in response to UV (from UV, UV-MG, and UV-SCX datasets) with sites whose ubiquitination is suppressed more than 2-fold by MLN4924 (from UV-MLN dataset). (D) Identification of proteins whose ubiquitination is stimulated by UV and inhibited by MLN4924. Blue bars represent Log2(L/H) ratios of diGly sites from UV-MG132 dataset (Figure 2A) while red bars represent ratios from UV-MLN dataset (Figure 6A). EXO1 represents a previously uncharacterized CRL substrate. (E) (Above) HeLa cells were released from a double-thymidine block and harvested at 2 hour intervals. Two hours prior to each point of harvest, cells were treated with 1 ιg/mL 4NQO. Propidium iodide stained cells from each time point were analyzed by flow cytometry, and lysates blotted for EXO1. (Below) HeLa cells were released from a double thymidine arrest and harvested 6 hours later. At 4 hours prior to harvest, they were treated with 5 ιM MLN4924 for 2 hours followed by 1 ιg/mL 4NQO for 2 hours. (F) HeLa cells infected with lentiviruses expressing dominant negative Cullins or empty vector (EV) were harvested 2 hours after 1 ιg/mL 4NQO treatment and blotted for EXO1. See also Figure S5.
Figure 7. SCF-Cyclin F mediates EXO1 ubiquitination in response to UV damage
(A) HA-tagged EXO1 was immunoprecipitated from 293T cells treated with 5 ιM MG132 for 30 minutes followed by 1 ιg/mL 4NQO for 2 hours. EXO1 complexes were eluted with HA peptide and interacting proteins identified by mass spectrometry. (B) 293T cells expressing HA-tagged EXO1 were treated with 5 ιM MG132 for 30 minutes followed by 1 ιg/mL 4NQO for 2 hours, and HA immunoprecipitates were blotted for Cyclin F. Asterisk represents a non-specific cross-reacting band. (C) HeLa cells transfected with siFF or siRNA pools against the F-box proteins SKP2, Cyclin F, FBXO18, or FBXW7 were treated with 1 ιg/mL 4NQO for 2 hours and lysates blotted for EXO1. (D, E) HeLa cells transfected with siFF or individual siRNAs against Cyclin F were treated with 1 ιg/mL 4NQO for 2 hours and lysates blotted for EXO1. EXO1 band intensities, measured by Image J, were normalized to tubulin or vinculin, and ratios from 4NQO to UNT (untreated) lanes calculated. Cyclin F mRNA depletion was confirmed by RT-qPCR. (F) HeLa cells transfected with siFF or siCCNF-4 were released from a double thymidine arrest and harvested 6 hours later. At 2 hours prior to harvest, they were treated with 1 ιg/mL 4NQO for 2 hours. See also Figure S6.
Similar articles
- Acetylation dynamics of human nuclear proteins during the ionizing radiation-induced DNA damage response.
Bennetzen MV, Larsen DH, Dinant C, Watanabe S, Bartek J, Lukas J, Andersen JS. Bennetzen MV, et al. Cell Cycle. 2013 Jun 1;12(11):1688-95. doi: 10.4161/cc.24758. Epub 2013 May 1. Cell Cycle. 2013. PMID: 23656789 Free PMC article. - VRK1 phosphorylates and protects NBS1 from ubiquitination and proteasomal degradation in response to DNA damage.
Monsalve DM, Campillo-Marcos I, Salzano M, Sanz-García M, Cantarero L, Lazo PA. Monsalve DM, et al. Biochim Biophys Acta. 2016 Apr;1863(4):760-9. doi: 10.1016/j.bbamcr.2016.02.005. Epub 2016 Feb 9. Biochim Biophys Acta. 2016. PMID: 26869104 - COP9 signalosome function in the DDR.
Hannss R, Dubiel W. Hannss R, et al. FEBS Lett. 2011 Sep 16;585(18):2845-52. doi: 10.1016/j.febslet.2011.04.027. Epub 2011 Apr 16. FEBS Lett. 2011. PMID: 21510940 Review. - DNA damage-induced proteasome phosphorylation controls substrate recognition and facilitates DNA repair.
Zhang X, Zhu T, Li X, Zhao H, Lin S, Huang J, Yang B, Guo X. Zhang X, et al. Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2321204121. doi: 10.1073/pnas.2321204121. Epub 2024 Aug 22. Proc Natl Acad Sci U S A. 2024. PMID: 39172782 - CRL4 Ubiquitin Pathway and DNA Damage Response.
Zhou P, Yan F. Zhou P, et al. Adv Exp Med Biol. 2020;1217:225-239. doi: 10.1007/978-981-15-1025-0_14. Adv Exp Med Biol. 2020. PMID: 31898231 Review.
Cited by
- HIV-1 Vpr Reprograms CLR4DCAF1 E3 Ubiquitin Ligase to Antagonize Exonuclease 1-Mediated Restriction of HIV-1 Infection.
Yan J, Shun MC, Hao C, Zhang Y, Qian J, Hrecka K, DeLucia M, Monnie C, Ahn J, Skowronski J. Yan J, et al. mBio. 2018 Oct 23;9(5):e01732-18. doi: 10.1128/mBio.01732-18. mBio. 2018. PMID: 30352932 Free PMC article. - Ubiquitination in T-Cell Activation and Checkpoint Inhibition: New Avenues for Targeted Cancer Immunotherapy.
Gavali S, Liu J, Li X, Paolino M. Gavali S, et al. Int J Mol Sci. 2021 Oct 6;22(19):10800. doi: 10.3390/ijms221910800. Int J Mol Sci. 2021. PMID: 34639141 Free PMC article. Review. - Beyond HAT Adaptor: TRRAP Liaisons with Sp1-Mediated Transcription.
Yin BK, Wang ZQ. Yin BK, et al. Int J Mol Sci. 2021 Nov 18;22(22):12445. doi: 10.3390/ijms222212445. Int J Mol Sci. 2021. PMID: 34830324 Free PMC article. Review. - Integrative proteomics reveals an increase in non-degradative ubiquitylation in activated CD4+ T cells.
Dybas JM, O'Leary CE, Ding H, Spruce LA, Seeholzer SH, Oliver PM. Dybas JM, et al. Nat Immunol. 2019 Jun;20(6):747-755. doi: 10.1038/s41590-019-0381-6. Epub 2019 May 6. Nat Immunol. 2019. PMID: 31061531 Free PMC article. - Discovery and Characterization of ZUFSP/ZUP1, a Distinct Deubiquitinase Class Important for Genome Stability.
Kwasna D, Abdul Rehman SA, Natarajan J, Matthews S, Madden R, De Cesare V, Weidlich S, Virdee S, Ahel I, Gibbs-Seymour I, Kulathu Y. Kwasna D, et al. Mol Cell. 2018 Apr 5;70(1):150-164.e6. doi: 10.1016/j.molcel.2018.02.023. Epub 2018 Mar 22. Mol Cell. 2018. PMID: 29576527 Free PMC article.
References
- Altmeyer M, Toledo L, Gudjonsson T, Grofte M, Rask MB, Lukas C, Akimov V, Blagoev B, Bartek J, Lukas J. The chromatin scaffold protein SAFB1 renders chromatin permissive for DNA damage signaling. Mol Cell. 2013;52:206–220. - PubMed
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
- Bekker-Jensen S, Mailand N. The ubiquitin- and SUMO-dependent signaling response to DNA double-strand breaks. FEBS Lett. 2011;585:2914–2919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM44664/GM/NIGMS NIH HHS/United States
- R01 GM044664/GM/NIGMS NIH HHS/United States
- R01 AG011085/AG/NIA NIH HHS/United States
- Howard Hughes Medical Institute/United States
- K12 CA087723/CA/NCI NIH HHS/United States
- R01 GM067945/GM/NIGMS NIH HHS/United States
- GM67945/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous