Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization - PubMed (original) (raw)
Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization
Di Fan et al. Nat Med. 2015 Jul.
Abstract
Candida albicans colonization is required for invasive disease. Unlike humans, adult mice with mature intact gut microbiota are resistant to C. albicans gastrointestinal (GI) colonization, but the factors that promote C. albicans colonization resistance are unknown. Here we demonstrate that commensal anaerobic bacteria-specifically clostridial Firmicutes (clusters IV and XIVa) and Bacteroidetes-are critical for maintaining C. albicans colonization resistance in mice. Using Bacteroides thetaiotamicron as a model organism, we find that hypoxia-inducible factor-1α (HIF-1α), a transcription factor important for activating innate immune effectors, and the antimicrobial peptide LL-37 (CRAMP in mice) are key determinants of C. albicans colonization resistance. Although antibiotic treatment enables C. albicans colonization, pharmacologic activation of colonic Hif1a induces CRAMP expression and results in a significant reduction of C. albicans GI colonization and a 50% decrease in mortality from invasive disease. In the setting of antibiotics, Hif1a and Camp (which encodes CRAMP) are required for B. thetaiotamicron-induced protection against C. albicans colonization of the gut. Thus, modulating C. albicans GI colonization by activation of gut mucosal immune effectors may represent a novel therapeutic approach for preventing invasive fungal disease in humans.
Figures
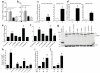
Figure 1. Candida spp. gastrointestinal colonization levels in antibiotic-treated adult mice, germ-free adult mice, and infant-adolescent mice
CA SC5314 colonization levels in (a) adult C57/BL6 mice (Harlan) pre-treated with (streptomycin, STREP; penicillin G, PCN; penicillin and streptomycin, PS; clindamycin, C; or metronidazole, M) or without antibiotics and then oral gavaged with CA. Mice continued on antibiotic water throughout the experiment. n=8. (b) Adult germ-free mice (C57/BL6). n=4. (c) Postnatal day (P)14, P28, and P42 mice (C57/BL6, Harlan). n=4. (d, e) Colonization levels of (d) other CA strains and (e) other Candida spp. Adult C3H/HeN mice (Harlan) pre-treated with or without antibiotics in the drinking water for 5 days then oral gavaged with (d) CA or other (e) Candida spp. Mice continued on antibiotic water throughout the experiment. n=4. Candida levels were measured every 7 days (a-c) or 21 days (d-e) after oral gavage. Points represent results from individual animals. Horizontal lines with bars represent the median with interquartile range.
Figure 2. Clostridial Firmicutes and Bacteroidetes Promote Candida albicans GI Colonization Resistance
(a) CA colonization levels of mice pre-treated with antibiotics and oral gavaged with CA. CA levels measured 7 days after oral gavage. n=8. (b) Relative abundance of bacterial Phyla as determined by 16S rRNA sequencing of fecal specimens collected from C3H/HeN mice treated with sterile water (WT), STREP, PCN, or PS. n=3. (c) Bacterial group qPCR (copies/g feces) performed on fecal gDNA collected from mice treated with sterile water or oral antibiotics. n=4. (d) CA colonization levels and (e) bacterial group qPCR in antibiotic-treated CA-colonized mice after cessation of oral antibiotics. Antibiotic water was discontinued on Day 0. CA levels and bacterial group qPCR measured every 7 days. n=4-5. (f) Bacterial (black circles) and CA (red triangle) levels in antibiotic-treated mice colonized with CA and gavaged with one bacterial commensal species. Colonization levels measured 14 days after bacterial gavage. n=8. Bacterial and CA levels in germ-free mice co-colonized with (g) Blautia producta and CA or (h) Bacteroides thetaiotamicron and CA. n=4. For all experiments, points represent results from individual animals. Horizontal lines represent the median with interquartertile range (a, d, f, g, h). Bars represent the mean with SEM (c, e). * p<0.05, ** p<0.01, *** p<0.001 Statistical analysis by Mann-Whitney test.
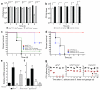
Figure 3. Bacteroidetes thetaiotamicron induces Hif1a and Cramp in mouse colons
(a) Hif1a and (b) Cramp mRNA expression in colons resected from CA colonization resistant mice (WT or STREP) and CA susceptible mice (PCN or PS). n=4. (c) _HIF1a_-Hif1a and (d) _LL-37_-Cramp mRNA expression measured in cultured human colonocytes exposed to ± CA and colons of antibiotic-treated mice ± CA colonization. n=4. (e) Hif1a and (f) Cramp mRNA expression measured in the colons of antibiotic treated mice ± oral gavage with commensal bacteria. n=4 (g) A representative western blot using an anti-CRAMP antibody (amino acids 135-173) against protein extracts from the distal colon of wild-type, Cramp KO, and antibiotic-treated mice ± oral gavage with commensal bacteria. Synthetic CRAMP peptide (5 ng, amino acids 135-173, 4.419 kDa) was used as a positive control. Cramp KO protein extracts were used as a negative control. Actin used as a loading control. Mouse CRAMP (amino acids 28-173) has a molecular mass of 16.422 kDa. A non-specific band (&) ~25kDa was detected in all lanes loaded with mouse colon protein extract. (f) Quantitative western blot analysis. Values obtained for CRAMP immunoblots were normalized to the optical density of corresponding immunoblots for actin. n=4. (i) Hif1a and (j) Cramp mRNA expression measured in the distal colon of germ-free mice colonized with CA, B. theta, or B. theta and CA. For all experiments, n=4. All data shown are means ± SEM. Statistical analysis by Mann-Whitney test. * p< 0.05; ** p<0.01; ns, not significant.
Figure 4. L-mimosine activation of HIf1a and Cramp in vivo decreases Candida albicans GI colonization and dissemination
Candida albicans GI colonization levels in (a) Hif1afl/fl, Hif1afl/fl Vil-Cre+, and (b) Cramp KO mice treated with antibiotics, colonized with CA and then treated ± L-mimosine. n=8. Bars represent the mean ± SEM. Statistical analysis performed by Mann-Whitney test. * p<0.05; ** p<0.001; ns, not significant. Survival curves of (c) Hif1afl/fl, Hif1afl/fl Vil-Cre+, and (d) Cramp KO mice treated with antibiotic water, colonized with CA, treated ± L-mimosine for five days and then given cyclophosphamide. L-mimosine treatment continued for an additional 7 days after the first cyclophosphamide dose. n=8. Statistical analysis performed by log-rank test. * p< 0.05; ** p<0.01; ns, not significant. (e) Hif1a and Cramp mRNA levels in colons of Hif1afl/fl, Hif1afl/fl Vil-Cre+, and Cramp KO mice treated ± L-mimosine. n=4. All data shown are means ± SEM. Assays were performed in triplicate. Statistical analysis performed by Mann-Whitney test. * p< 0.05; ** p<0.01; ns, not significant. (g) C. albicans (red triangles) and B. theta (black circles) GI colonization levels in antibiotic-treated Hif1afl/fl, Hif1afl/fl Vil-Cre+, and Cramp KO mice. n= 6 for Hif1afl/fl and Cramp KO mice. n=5 for Hif1afl/fl Vil-Cre+. Black circles (B. theta) and red triangles (CA) represent results from individual animals. Horizontal lines represent the median with interquartertile range. * p<0.05, ** p<0.01. Statistical analysis by Mann-Whitney test.
Comment in
- The complexities of bacterial-fungal interactions in the mammalian gastrointestinal tract.
Lopez-Medina E, Koh AY. Lopez-Medina E, et al. Microb Cell. 2016 Mar 16;3(5):191-195. doi: 10.15698/mic2016.05.497. Microb Cell. 2016. PMID: 28357354 Free PMC article. No abstract available.
Similar articles
- Horizontal transmission of Candida albicans and evidence of a vaccine response in mice colonized with the fungus.
Cutler JE, Corti M, Lambert P, Ferris M, Xin H. Cutler JE, et al. PLoS One. 2011;6(7):e22030. doi: 10.1371/journal.pone.0022030. Epub 2011 Jul 19. PLoS One. 2011. PMID: 21818288 Free PMC article. - Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract.
Matsuo K, Haku A, Bi B, Takahashi H, Kamada N, Yaguchi T, Saijo S, Yoneyama M, Goto Y. Matsuo K, et al. Microbiol Immunol. 2019 May;63(5):155-163. doi: 10.1111/1348-0421.12680. Epub 2019 May 15. Microbiol Immunol. 2019. PMID: 30919462 - Mucosal damage and neutropenia are required for Candida albicans dissemination.
Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Koh AY, et al. PLoS Pathog. 2008 Feb 8;4(2):e35. doi: 10.1371/journal.ppat.0040035. PLoS Pathog. 2008. PMID: 18282097 Free PMC article. - The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine.
Kumamoto CA, Gresnigt MS, Hube B. Kumamoto CA, et al. Curr Opin Microbiol. 2020 Aug;56:7-15. doi: 10.1016/j.mib.2020.05.006. Epub 2020 Jun 27. Curr Opin Microbiol. 2020. PMID: 32604030 Free PMC article. Review. - Interplay between Candida albicans and the antimicrobial peptide armory.
Swidergall M, Ernst JF. Swidergall M, et al. Eukaryot Cell. 2014 Aug;13(8):950-7. doi: 10.1128/EC.00093-14. Epub 2014 Jun 20. Eukaryot Cell. 2014. PMID: 24951441 Free PMC article. Review.
Cited by
- Prevalence, Species Distribution and Resistance of Candidemia in Pediatric and Adult Patients in a Northeast Italy University Hospital.
Meneghello S, Bernabè G, Di Pietra G, Di Sopra S, Del Vecchio C, Cattelan AM, Castagliuolo I, Brun P. Meneghello S, et al. J Fungi (Basel). 2024 Oct 10;10(10):707. doi: 10.3390/jof10100707. J Fungi (Basel). 2024. PMID: 39452659 Free PMC article. - New insights into the intestinal barrier through "gut-organ" axes and a glimpse of the microgravity's effects on intestinal barrier.
Nie HY, Ge J, Huang GX, Liu KG, Yue Y, Li H, Lin HG, Zhang T, Yan HF, Xu BX, Sun HW, Yang JW, Si SY, Zhou JL, Cui Y. Nie HY, et al. Front Physiol. 2024 Oct 10;15:1465649. doi: 10.3389/fphys.2024.1465649. eCollection 2024. Front Physiol. 2024. PMID: 39450142 Free PMC article. Review. - Effect of diet on the gut mycobiome and potential implications in inflammatory bowel disease.
Buttar J, Kon E, Lee A, Kaur G, Lunken G. Buttar J, et al. Gut Microbes. 2024 Jan-Dec;16(1):2399360. doi: 10.1080/19490976.2024.2399360. Epub 2024 Sep 17. Gut Microbes. 2024. PMID: 39287010 Free PMC article. Review. - Interplay between Bile Acids and Intestinal Microbiota: Regulatory Mechanisms and Therapeutic Potential for Infections.
Li W, Chen H, Tang J. Li W, et al. Pathogens. 2024 Aug 20;13(8):702. doi: 10.3390/pathogens13080702. Pathogens. 2024. PMID: 39204302 Free PMC article. Review. - Cancer and the Microbiome of the Human Body.
Herrera-Quintana L, Vázquez-Lorente H, Lopez-Garzon M, Cortés-Martín A, Plaza-Diaz J. Herrera-Quintana L, et al. Nutrients. 2024 Aug 21;16(16):2790. doi: 10.3390/nu16162790. Nutrients. 2024. PMID: 39203926 Free PMC article. Review.
References
- Miranda LN, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. - PubMed
- Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–1967. - PubMed
- Pizzo PA, Poplack D, editors. Principles and Practice of Pediatric Oncology. 6th Edition Lippincott Williams & Wilkins; Philadelphia: 2011. p. 1531.
Methods-only References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK060855/DK/NIDDK NIH HHS/United States
- R01 DK070855/DK/NIDDK NIH HHS/United States
- P30CA142543/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- P50 CA070907/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases