Sigma receptors [σRs]: biology in normal and diseased states - PubMed (original) (raw)
Sigma receptors [σRs]: biology in normal and diseased states
Colin G Rousseaux et al. J Recept Signal Transduct Res. 2016 Aug.
Abstract
This review compares the biological and physiological function of Sigma receptors [σRs] and their potential therapeutic roles. Sigma receptors are widespread in the central nervous system and across multiple peripheral tissues. σRs consist of sigma receptor one (σ1R) and sigma receptor two (σ2R) and are expressed in numerous regions of the brain. The sigma receptor was originally proposed as a subtype of opioid receptors and was suggested to contribute to the delusions and psychoses induced by benzomorphans such as SKF-10047 and pentazocine. Later studies confirmed that σRs are non-opioid receptors (not an µ opioid receptor) and play a more diverse role in intracellular signaling, apoptosis and metabolic regulation. σ1Rs are intracellular receptors acting as chaperone proteins that modulate Ca2+ signaling through the IP3 receptor. They dynamically translocate inside cells, hence are transmembrane proteins. The σ1R receptor, at the mitochondrial-associated endoplasmic reticulum membrane, is responsible for mitochondrial metabolic regulation and promotes mitochondrial energy depletion and apoptosis. Studies have demonstrated that they play a role as a modulator of ion channels (K+ channels; N-methyl-d-aspartate receptors [NMDAR]; inositol 1,3,5 triphosphate receptors) and regulate lipid transport and metabolism, neuritogenesis, cellular differentiation and myelination in the brain. σ1R modulation of Ca2+ release, modulation of cardiac myocyte contractility and may have links to G-proteins. It has been proposed that σ1Rs are intracellular signal transduction amplifiers. This review of the literature examines the mechanism of action of the σRs, their interaction with neurotransmitters, pharmacology, location and adverse effects mediated through them.
Keywords: Apoptosis; cannabinoids; central nervous system; glutamate; neoplasia; non-opioid receptors.
Figures
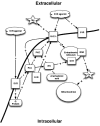
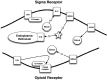
Figure 1.
σRs and their effect on intracellular calcium concentrations. PLC – phospholipases C; PKC – protein kinase C; S1R – sigma1; IP3 – inositol triphosphate; IP3R – inositol triphosphate receptor; NMDA – N-methyl-D-aspartate receptor; Mg – magnesium; Ca – calcium.
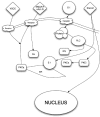
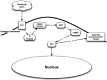
Figure 2.
Putative biological action of the σ1R on neuronal function. PLC – phospholipases C; PKCa – protein kinase C alpha; PKCi – protein kinase C inhibitor; S 1 – sigma1 receptor; IP3 – inositol triphosphate; nAch – nicotinic acetylcholine; nAchR – nicotinic acetylcholine receptor; NMDAR – N-methyl-d-aspartate receptors; Ca – calcium; VSCC – voltage-sensitive calcium channels. Once a neuron has been activated, e.g. via Glu or acetylcholine, a concomitant influx of Ca2+ and [Ca2+]i mobilization occur, facilitated by the activation of the endoplasmic-reticulum-bound σ1R, which is also triggered by numerous xenobiotics and steroids. The subsequent activation of PLC and the recruitment of the PKCs from its inactive form [PKC_i_] to its active form [PKCa], which is translocated to the plasma membrane, result in the activation of various enzymatic processes, as well as the phosphorylation of membrane-bound neurotransmitter receptors. In turn, the σ1R translocates to the plasma membrane where it decreases the excitatory neurotransmitter-induced Ca2+ influx.
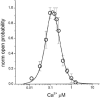
Figure 3.
Bell curve dose response. Bell-shaped Ca2+ dependence of recombinant IP3R. Recombinant IP3R activity was measured in bilayers in the presence of 2 µM InsP3 and 1 mM Na2ATP at cis (cytosolic) Ca2+ concentrations in the range between 10 nM and 5 µM Ca2+. Ca2+ concentration in the cis chamber was adjusted by using calibrated 20 mM CaCl2 stock solution and 1 mM mixture of HEDTA and EGTA. Po in each experiment was normalized to maximum Po observed in the same experiment, and then data from three independent experiments were averaged together at each Ca2+ concentration (○) (477).
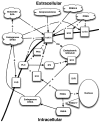
Figure 4.
Neurosteroids and their interactions with σRs. PLC – phospholipases C; PKC – protein kinase C; PKCi – protein kinase C inhibitor; S1R – sigma1 receptor; IP3 – inositol triphosphate; EAA – excitotoxic amino acid; GABA – γ-aminobutyric acid; NMDA – N-methyl-
d
-aspartate receptor; Ca – calcium; DHEA – dihydroepiandrosterone; PREG – pregnenolone; PREG-S – pregnenolone sulfate ester; P450c17 – cytochrome P450 C17.
Figure 5.
A schematic representation of the opioid receptor and σ1Rs. NMDA – N-methyl-
d
-aspartate receptor; K+C – potassium channel; ! – increased concentration; AChR – acetylcholine receptor; S1R – σ1R; Ca2+ – calcium; K+ – potassium.
Figure 6.
Serotonin (5HT) stimulation of the σ1R. PKA – phosphokinase A; ATP – adenosine triphosphate; cAMP – cyclic adenosine monophosphate; S1R – σ1R.
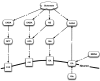
Figure 7.
Interaction of glutamate, neurotransmitters and the σR. NMDA – N-methyl-
d
-aspartate receptor; NE – norepinephrine; NPY – neuropeptide Y; ACh – acetylcholine; M3 – rat muscarinic acetyl choline receptor; GABA – γ-aminobutyric acid; GA – Ga-binding protein α-chain; Ka – kainate; Glu – glutamate; S1R – σ1R.
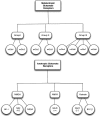
Figure 8.
Glutamate receptors types. NMDA – N-methyl-
d
-aspartate receptor; AMPA – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; KA – kainate; GluR – glutamate receptor; NR – NMDA receptor subtype.
Figure 9.
The basic mechanism of neuroprotection by σ1R agonists.
Similar articles
- Mechanisms of activation of nucleus accumbens neurons by cocaine via sigma-1 receptor-inositol 1,4,5-trisphosphate-transient receptor potential canonical channel pathways.
Barr JL, Deliu E, Brailoiu GC, Zhao P, Yan G, Abood ME, Unterwald EM, Brailoiu E. Barr JL, et al. Cell Calcium. 2015 Aug;58(2):196-207. doi: 10.1016/j.ceca.2015.05.001. Epub 2015 May 27. Cell Calcium. 2015. PMID: 26077147 Free PMC article. - The ON:OFF switch, σ1R-HINT1 protein, controls GPCR-NMDA receptor cross-regulation: implications in neurological disorders.
Rodríguez-Muñoz M, Cortés-Montero E, Pozo-Rodrigálvarez A, Sánchez-Blázquez P, Garzón-Niño J. Rodríguez-Muñoz M, et al. Oncotarget. 2015 Nov 3;6(34):35458-77. doi: 10.18632/oncotarget.6064. Oncotarget. 2015. PMID: 26461475 Free PMC article. - The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control.
Rodríguez-Muñoz M, Sánchez-Blázquez P, Herrero-Labrador R, Martínez-Murillo R, Merlos M, Vela JM, Garzón J. Rodríguez-Muñoz M, et al. Antioxid Redox Signal. 2015 Apr 1;22(10):799-818. doi: 10.1089/ars.2014.5993. Epub 2015 Feb 18. Antioxid Redox Signal. 2015. PMID: 25557043 Free PMC article. - Early development of sigma-receptor ligands.
Narayanan S, Bhat R, Mesangeau C, Poupaert JH, McCurdy CR. Narayanan S, et al. Future Med Chem. 2011 Jan;3(1):79-94. doi: 10.4155/fmc.10.279. Future Med Chem. 2011. PMID: 21428827 Review.
Cited by
- A Systematic Review of Oral Vertical Dyskinesia ("Rabbit" Syndrome).
Rissardo JP, Kherajani K, Vora NM, Yatakarla V, Fornari Caprara AL, Ratliff J, Caroff SN. Rissardo JP, et al. Medicina (Kaunas). 2024 Aug 19;60(8):1347. doi: 10.3390/medicina60081347. Medicina (Kaunas). 2024. PMID: 39202628 Free PMC article. - Amantadine for Traumatic Brain Injury-Supporting Evidence and Mode of Action.
Dekundy A, Pichler G, El Badry R, Scheschonka A, Danysz W. Dekundy A, et al. Biomedicines. 2024 Jul 13;12(7):1558. doi: 10.3390/biomedicines12071558. Biomedicines. 2024. PMID: 39062131 Free PMC article. Review. - Favorable efficacy and reduced acute neurotoxicity by antisense oligonucleotides with 2',4'-BNA/LNA with 9-(aminoethoxy)phenoxazine.
Matsubayashi T, Yoshioka K, Lei Mon SS, Katsuyama M, Jia C, Yamaguchi T, Hara RI, Nagata T, Nakagawa O, Obika S, Yokota T. Matsubayashi T, et al. Mol Ther Nucleic Acids. 2024 Mar 18;35(2):102161. doi: 10.1016/j.omtn.2024.102161. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38978695 Free PMC article. - Design, Synthesis, and Cytotoxic Assessment of New Haloperidol Analogues as Potential Anticancer Compounds Targeting Sigma Receptors.
Zampieri D, Romano M, Fortuna S, Amata E, Dichiara M, Cosentino G, Marrazzo A, Mamolo MG. Zampieri D, et al. Molecules. 2024 Jun 6;29(11):2697. doi: 10.3390/molecules29112697. Molecules. 2024. PMID: 38893570 Free PMC article. - Fenfluramine increases survival and reduces markers of neurodegeneration in a mouse model of Dravet syndrome.
Cha J, Filatov G, Smith SJ, Gammaitoni AR, Lothe A, Reeder T. Cha J, et al. Epilepsia Open. 2024 Feb;9(1):300-313. doi: 10.1002/epi4.12873. Epub 2023 Dec 22. Epilepsia Open. 2024. PMID: 38018342 Free PMC article.
References
- Martin WR, Eades CG, Thompson JA, et al. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharm Exp Therap. 1976;197:517–32. - PubMed
- Pan YX, Mei J, Xu J, et al. Cloning and characterization of a mouse sigma1 receptor. J Neurochem. 1998;70:2279–85. - PubMed
- Prasad PD, Srinivas SR, Wang H, et al. Electrogenic nature of rat sodium-dependent multivitamin transport. Biochem Biophys Res Commun. 2000;270:836–40. - PubMed
- Seth P, Fei YJ, Li HW, et al. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–31. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous