The extracellular matrix and insulin resistance - PubMed (original) (raw)
Review
The extracellular matrix and insulin resistance
Ashley S Williams et al. Trends Endocrinol Metab. 2015 Jul.
Abstract
The extracellular matrix (ECM) is a highly-dynamic compartment that undergoes remodeling as a result of injury and repair. Over the past decade, mounting evidence in humans and rodents suggests that ECM remodeling is associated with diet-induced insulin resistance in several metabolic tissues. In addition, integrin receptors for the ECM have also been implicated in the regulation of insulin action. This review addresses what is currently known about the ECM, integrins, and insulin action in the muscle, liver, and adipose tissue. Understanding how ECM remodeling and integrin signaling regulate insulin action may aid in the development of new therapeutic targets for the treatment of insulin resistance and type 2 diabetes (T2D).
Keywords: extracellular matrix; glucose homeostasis; insulin resistance; integrins; liver; muscle.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
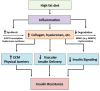
Figure 1
A link between extracellular matrix remodeling and insulin resistance. A diet high in fat generates a state of chronic inflammation. This inflammatory response leads to increased ECM synthesis and decreased ECM degradation, resulting in increased deposition and remodeling of ECM. Increased levels of ECM lead to increased physical barriers for insulin and glucose transport, decreased vascular insulin delivery and decreased insulin signaling. The combination of all of these factors then culminates in insulin resistance.
Figure 2
The role of integrin α2β1 diet-induced muscle insulin resistance. In the HF-fed state, capillary density and endothelial function are impaired. This results in decreased potential for glucose and insulin transport into the interstitial space despite hyperglycemia and hyperinsulinemia. Moreover, increased ECM deposition in the interstitial space also provides a physical barrier to glucose and insulin transport to the myocyte. Insulin signaling within the myocyte is impaired and this may be attributed to increased integrin α2β1 signaling as a consequence of increased deposition of the ECM. This results in impaired Glut4 translocation and decreased glucose transport into the myocyte. In contrast, the genetic deletion of the integrin α2 subunit results in improved insulin-stimulated muscle glucose uptake.
Figure 3
The role of integrin α1β1 in diet-induced hepatic insulin resistance. In the HF-fed state, sinusoidal capillarization occurs and this, in addition to increased ECM buildup in the space of Disse, results in decreased insulin transport to the hepatocyte despite hyperinsulinemia. Protein expression of the integrin α1 subunit is increased and this leads to increased α1β1 cell signaling. Upon insulin stimulation, the combination of both insulin and integrin α1β1 signaling results in some insulin signaling and the partial suppression of hepatic glucose output. In contrast, the genetic deletion of the integrin α1 subunit results in severe hepatic insulin resistance and no insulin-mediated suppression of hepatic glucose output. This is attributed to decreased insulin signaling. It is possible that this effect is mediated by integrin α5β1, the only other known integrin expressed on the hepatocyte, however this is currently unknown.
Figure 4
Proposed model whereby integrins regulate insulin action. In the presence of insulin, integrin signaling through both integrin linked kinase (ILK) and focal adhesion kinase (FAK) promotes insulin action. Canonical insulin signaling occurs, however it is possible that other mechanisms exist whereby insulin exerts its actions within the cell. Several studies show that FAK is an important regulator of insulin action in both the muscle and liver. Less is known about ILK. However, Nck2 is an adaptor protein shared by both the insulin receptor and ILK. This suggests that there may be a physical link between the insulin receptor and integrins through Nck2 and ILK, allowing the centralization of signaling through this complex. Akt, a critical insulin signaling molecule, is a known binding partner of ILK. Additionally, integrin signaling has been shown to modulate the assembly of the cytoskeleton and this may have effects on both mitochondrial function and insulin action.
Similar articles
- Adipose extracellular matrix remodelling in obesity and insulin resistance.
Lin D, Chun TH, Kang L. Lin D, et al. Biochem Pharmacol. 2016 Nov 1;119:8-16. doi: 10.1016/j.bcp.2016.05.005. Epub 2016 May 12. Biochem Pharmacol. 2016. PMID: 27179976 Free PMC article. Review. - Implications of Skeletal Muscle Extracellular Matrix Remodeling in Metabolic Disorders: Diabetes Perspective.
Ahmad K, Choi I, Lee YH. Ahmad K, et al. Int J Mol Sci. 2020 May 28;21(11):3845. doi: 10.3390/ijms21113845. Int J Mol Sci. 2020. PMID: 32481704 Free PMC article. Review. - Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases.
Ruiz-Ojeda FJ, Méndez-Gutiérrez A, Aguilera CM, Plaza-Díaz J. Ruiz-Ojeda FJ, et al. Int J Mol Sci. 2019 Oct 2;20(19):4888. doi: 10.3390/ijms20194888. Int J Mol Sci. 2019. PMID: 31581657 Free PMC article. Review. - Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice.
Kang L, Ayala JE, Lee-Young RS, Zhang Z, James FD, Neufer PD, Pozzi A, Zutter MM, Wasserman DH. Kang L, et al. Diabetes. 2011 Feb;60(2):416-26. doi: 10.2337/db10-1116. Diabetes. 2011. PMID: 21270253 Free PMC article. - Obesity and type 2 diabetes mellitus: insights from skeletal muscle extracellular matrix remodeling.
Wu L, Coletta DK. Wu L, et al. Am J Physiol Cell Physiol. 2025 Jun 1;328(6):C1752-C1763. doi: 10.1152/ajpcell.00154.2024. Epub 2025 Apr 17. Am J Physiol Cell Physiol. 2025. PMID: 40244268 Review.
Cited by
- Integrin-Src-YAP1 signaling mediates the melanoma acquired resistance to MAPK and PI3K/mTOR dual targeted therapy.
Yu C, Zhang M, Song J, Zheng X, Xu G, Bao Y, Lan J, Luo D, Hu J, Li JJ, Shi H. Yu C, et al. Mol Biomed. 2020 Nov 10;1(1):12. doi: 10.1186/s43556-020-00013-0. Mol Biomed. 2020. PMID: 35006410 Free PMC article. - Regulation of the dystrophin-associated glycoprotein complex composition by the metabolic properties of muscle fibres.
Omairi S, Hau KL, Collins-Hooper H, Scott C, Vaiyapuri S, Torelli S, Montanaro F, Matsakas A, Patel K. Omairi S, et al. Sci Rep. 2019 Feb 26;9(1):2770. doi: 10.1038/s41598-019-39532-4. Sci Rep. 2019. PMID: 30808964 Free PMC article. - Adapting to obesity with adipose tissue inflammation.
Reilly SM, Saltiel AR. Reilly SM, et al. Nat Rev Endocrinol. 2017 Nov;13(11):633-643. doi: 10.1038/nrendo.2017.90. Epub 2017 Aug 11. Nat Rev Endocrinol. 2017. PMID: 28799554 Review. - Integration of metabolic flux with hepatic glucagon signaling and gene expression profiles in the conscious dog.
Coate KC, Ramnanan CJ, Smith M, Winnick JJ, Kraft G, Irimia-Dominguez J, Farmer B, Donahue EP, Roach PJ, Cherrington AD, Edgerton DS. Coate KC, et al. Am J Physiol Endocrinol Metab. 2024 Apr 1;326(4):E428-E442. doi: 10.1152/ajpendo.00316.2023. Epub 2024 Feb 7. Am J Physiol Endocrinol Metab. 2024. PMID: 38324258 Free PMC article. - Microenvironmental Control of Adipocyte Fate and Function.
Pope BD, Warren CR, Parker KK, Cowan CA. Pope BD, et al. Trends Cell Biol. 2016 Oct;26(10):745-755. doi: 10.1016/j.tcb.2016.05.005. Epub 2016 Jun 4. Trends Cell Biol. 2016. PMID: 27268909 Free PMC article. Review.
References
- Martinez-Hernandez A, et al. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. - PubMed
- Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. - PubMed
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
- Moser M, et al. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK050277/DK/NIDDK NIH HHS/United States
- DK54902/DK/NIDDK NIH HHS/United States
- DK050277/DK/NIDDK NIH HHS/United States
- DK059637/DK/NIDDK NIH HHS/United States
- R01 DK050277/DK/NIDDK NIH HHS/United States
- R56 DK054902/DK/NIDDK NIH HHS/United States
- R01 DK054902/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous