CRMP4 and CRMP2 Interact to Coordinate Cytoskeleton Dynamics, Regulating Growth Cone Development and Axon Elongation - PubMed (original) (raw)
CRMP4 and CRMP2 Interact to Coordinate Cytoskeleton Dynamics, Regulating Growth Cone Development and Axon Elongation
Minghui Tan et al. Neural Plast. 2015.
Abstract
Cytoskeleton dynamics are critical phenomena that underpin many fundamental cellular processes. Collapsin response mediator proteins (CRMPs) are highly expressed in the developing nervous system, mediating growth cone guidance, neuronal polarity, and axonal elongation. However, whether and how CRMPs associate with microtubules and actin coordinated cytoskeletal dynamics remain unknown. In this study, we demonstrated that CRMP2 and CRMP4 interacted with tubulin and actin in vitro and colocalized with the cytoskeleton in the transition-zone in developing growth cones. CRMP2 and CRMP4 also interacted with one another coordinately to promote growth cone development and axonal elongation. Genetic silencing of CRMP2 enhanced, whereas overexpression of CRMP2 suppressed, the inhibitory effects of CRMP4 knockdown on axonal development. In addition, knockdown of CRMP2 or overexpression of truncated CRMP2 reversed the promoting effect of CRMP4. With the overexpression of truncated CRMP2 or CRMP4 lacking the cytoskeleton interaction domain, the promoting effect of CRMP was suppressed. These data suggest a model in which CRMP2 and CRMP4 form complexes to bridge microtubules and actin and thus work cooperatively to regulate growth cone development and axonal elongation.
Figures
Figure 1
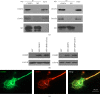
CRMP2 and CRMP4 interact with tubulin and actin. (a) Bacterial recombinant glutathione S-transferase- (GST-) CRMPs were purified and subjected to GST-pulldown assays with growth cone extracts from rat brain. Coomassie blue staining of GST and GST-CRMPs (bottom) showed equal loading of bait proteins. The pulldown sediments were subjected to western blot assays with tubulin and actin antibodies using GAPDH as the lysate input control. Each result is representative of three to five separate experiments with similar results. (b) Growth cone lysates from rat brain were subjected to coimmunoprecipitation (Co-IP) with tubulin or actin antibody and then processed for western blot (WB) analysis with the indicated antibodies. (c) Growth cones lysates were immunoprecipitated with CRMP2 or CRMP4 antibodies and processed for WB assays to detect the indicated proteins using the appropriate antibodies.
Figure 2
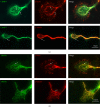
CRMP2 and CRMP4 colocalize with tubulin and actin in growth cones. (a) Anti-CRMP4, anti-tubulin, and anti-actin antibodies were used to detect endogenous proteins in the growth cones of hippocampal neurons. (b) Anti-CRMP2, anti-tubulin, and anti-actin antibodies were used to detect endogenous proteins in the growth cones of hippocampal neurons. Scale bar: 10 _μ_m.
Figure 3
CRMP2 and CRMP4 form complexes in growth cones. (a) Growth cones lysates from rat brain were subjected to glutathione S-transferase- (GST-) pulldown assays using GST-CRMP2 or GST-CRMP4. The retrieved sediments were subjected to western blot analysis using the CRMP4 or CRMP2 antibodies. The GAPDH antibody was used to show equal loading. (b) Growth cones lysates from rat brain were subjected to coimmunoprecipitation (Co-IP) assays with rabbit CRMP2 and CRMP4 antibodies, and the resulting sediments were subjected to western blot analysis with mouse CRMP4 or CRMP2 antibodies. (c) Rabbit anti-CRMP2 and mouse anti-CRMP4 antibodies were used to detect endogenous proteins in the growth cones of hippocampal neurons. Scale bar: 10 _μ_m.
Figure 4
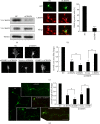
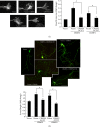
Both CRMP2 and CRMP4 are necessary for growth cone development and axonal elongation, and CRMP2 rescues the inhibitory effect of CRMP4 knockdown. (a) The efficiency of CRMP2 siRNA was measured in HEK293 cells by transfection with a nontargeting siRNA (negative control, NC) or the siCRMP4 fragment together with the CRMP4-V5 plasmid. Cell lysates of HEK293 cells were analyzed using western blotting with the V5 antibody. Tubulin was used as a loading control. (b) Neurons at 1 day in vitro (DIV) were transfected with GFP together with the CRMP4 siRNA fragments or NC. Neurons were stained with anti-GFP (green) and anti-CRMP4 (red). Representative images are shown (left panel). The percentage of CRMP4-positive GFP-transfected neurons (right panel). Mean ± SEM, n = 3; ∗∗∗ P < 0.001 versus NC. (c) Representative images of growth cones from neurons of the indicated transfections. Scale bar: 10 _μ_m. The relative ratio of the growth cone area of transfected cells was measured and is shown in the right panel. Mean ± SEM, n = 3; ∗ P < 0.05 versus NC; # P < 0.05 versus indicated control; ∗∗ P < 0.01 versus NC. (d) Hippocampal neurons were transfected with indicated plasmids or siRNA fragments together with GFP. Neurons were fixed at 4 DIV and stained with a GFP antibody. Axon length was measured as the mean ± SEM from three independent experiments. ∗ P < 0.05 versus NC; # P < 0.05 versus indicated control. Scale bar: 100 _μ_m.
Figure 5
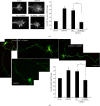
Genetic silencing of CRMP2 inhibits the promoting effect of CRMP4 on growth cone development and axonal elongation. (a) Representative images of growth cones with indicated transfections are shown. Scale bar: 10 _μ_m. Relative ratio of the growth cone area was measured and shown in the right panel. Mean ± SEM, n = 3; ∗ P < 0.05 versus vector control; # P < 0.05 versus NC. (b) Hippocampal neurons were transfected with the indicated plasmids or siRNA fragments together with GFP. Representative images of GFP staining are shown. Axon length was measured as the mean ± SEM from three independent experiments (n = 3). ∗ P < 0.05 versus vector control; # P < 0.05 versus NC. Scale bar: 100 _μ_m.
Figure 6
CRMP2 and CRMP4 interact with the cytoskeleton to promote growth cone development and axonal elongation. (a) Representative images of growth cones from neurons transfected with CRMP4 alone or together with CRMP2ΔC322 or with CRMP2 alone or together with CRMP4ΔC472 are shown. Scale bar: 10 _μ_m. The relative ratio of the growth cone area was measured and is shown in the right panel. Mean ± SEM, n = 4; ∗ P < 0.05 versus vector control; # P < 0.05 versus indicated group. (b) Representative images of GFP staining from hippocampal neurons with the same transfection as in (a) are shown. Axon length was measured as the mean ± SEM from three independent experiments. ∗ P < 0.05 versus vector control; ∗∗ P < 0.01 versus vector control; # P < 0.05 versus indicated group. Scale bar: 100 _μ_m.
Figure 7
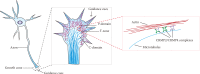
Diagram of a model for CRMP2 and CRMP4 coordinately regulating growth cone development and axon elongation via cytoskeleton dynamics. The schematic model shows that CRMP2 and CRMP4 may form complexes (homo- or heterotetramers) bridging microtubules and actin to mediate cytoskeleton dynamics, thus regulating growth cone development and axon elongation.
Similar articles
- Collapsin response mediator protein 4 regulates growth cone dynamics through the actin and microtubule cytoskeleton.
Khazaei MR, Girouard MP, Alchini R, Ong Tone S, Shimada T, Bechstedt S, Cowan M, Guillet D, Wiseman PW, Brouhard G, Cloutier JF, Fournier AE. Khazaei MR, et al. J Biol Chem. 2014 Oct 24;289(43):30133-43. doi: 10.1074/jbc.M114.570440. Epub 2014 Sep 15. J Biol Chem. 2014. PMID: 25225289 Free PMC article. - CRMP‑5 interacts with actin to regulate neurite outgrowth.
Gong X, Tan M, Gao Y, Chen K, Guo G. Gong X, et al. Mol Med Rep. 2016 Feb;13(2):1179-85. doi: 10.3892/mmr.2015.4662. Epub 2015 Dec 9. Mol Med Rep. 2016. PMID: 26677106 Free PMC article. - CRMP2 and CRMP4 Are Differentially Required for Axon Guidance and Growth in Zebrafish Retinal Neurons.
Liu ZZ, Zhu J, Wang CL, Wang X, Han YY, Liu LY, Xu HA. Liu ZZ, et al. Neural Plast. 2018 Jun 21;2018:8791304. doi: 10.1155/2018/8791304. eCollection 2018. Neural Plast. 2018. PMID: 30034463 Free PMC article. - Cytoskeletal dynamics in growth-cone steering.
Geraldo S, Gordon-Weeks PR. Geraldo S, et al. J Cell Sci. 2009 Oct 15;122(Pt 20):3595-604. doi: 10.1242/jcs.042309. J Cell Sci. 2009. PMID: 19812305 Free PMC article. Review. - Cytoskeletal social networking in the growth cone: How +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance.
Cammarata GM, Bearce EA, Lowery LA. Cammarata GM, et al. Cytoskeleton (Hoboken). 2016 Sep;73(9):461-76. doi: 10.1002/cm.21272. Epub 2016 Feb 8. Cytoskeleton (Hoboken). 2016. PMID: 26783725 Free PMC article. Review.
Cited by
- Probenecid Disrupts a Novel Pannexin 1-Collapsin Response Mediator Protein 2 Interaction and Increases Microtubule Stability.
Xu X, Wicki-Stordeur LE, Sanchez-Arias JC, Liu M, Weaver MS, Choi CSW, Swayne LA. Xu X, et al. Front Cell Neurosci. 2018 May 11;12:124. doi: 10.3389/fncel.2018.00124. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29867357 Free PMC article. - CRMP4-mediated fornix development involves Semaphorin-3E signaling pathway.
Boulan B, Ravanello C, Peyrel A, Bosc C, Delphin C, Appaix F, Denarier E, Kraut A, Jacquier-Sarlin M, Fournier A, Andrieux A, Gory-Fauré S, Deloulme JC. Boulan B, et al. Elife. 2021 Dec 3;10:e70361. doi: 10.7554/eLife.70361. Elife. 2021. PMID: 34860155 Free PMC article. - Contribution of the dihydropyrimidinase-like proteins family in synaptic physiology and in neurodevelopmental disorders.
Desprez F, Ung DC, Vourc'h P, Jeanne M, Laumonnier F. Desprez F, et al. Front Neurosci. 2023 Apr 17;17:1154446. doi: 10.3389/fnins.2023.1154446. eCollection 2023. Front Neurosci. 2023. PMID: 37144098 Free PMC article. Review. - Microtubule association of TRIM3 revealed by differential extraction proteomics.
Glover HL, Mendes M, Gomes-Neto J, Rusilowicz-Jones EV, Rigden DJ, Dittmar G, Urbé S, Clague MJ. Glover HL, et al. J Cell Sci. 2024 Jan 15;137(2):jcs261522. doi: 10.1242/jcs.261522. Epub 2024 Jan 31. J Cell Sci. 2024. PMID: 38149663 Free PMC article. - The oligodendrocyte growth cone and its actin cytoskeleton: A fundamental element for progenitor cell migration and CNS myelination.
Thomason EJ, Escalante M, Osterhout DJ, Fuss B. Thomason EJ, et al. Glia. 2020 Jul;68(7):1329-1346. doi: 10.1002/glia.23735. Epub 2019 Nov 7. Glia. 2020. PMID: 31696982 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous