Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research - PubMed (original) (raw)
Review
doi: 10.1016/j.plipres.2015.05.002. Epub 2015 Jun 11.
Isabel Veloso Alves Pereira 2, Michaël Maes 3, Sara Crespo Yanguas 4, Isabelle Colle 5, Bert Van Den Bossche 6, Tereza Cristina Da Silva 7, Cláudia Pinto Marques Souza de Oliveira 8, Wellington Andraus 9, Venâncio Avancini Alves 10, Bruno Cogliati 11, Mathieu Vinken 12
Affiliations
- PMID: 26073454
- PMCID: PMC4596006
- DOI: 10.1016/j.plipres.2015.05.002
Review
Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research
Joost Willebrords et al. Prog Lipid Res. 2015 Jul.
Abstract
Non-alcoholic fatty liver disease encompasses a spectrum of liver diseases, including simple steatosis, steatohepatitis, liver fibrosis and cirrhosis and hepatocellular carcinoma. Non-alcoholic fatty liver disease is currently the most dominant chronic liver disease in Western countries due to the fact that hepatic steatosis is associated with insulin resistance, type 2 diabetes mellitus, obesity, metabolic syndrome and drug-induced injury. A variety of chemicals, mainly drugs, and diets is known to cause hepatic steatosis in humans and rodents. Experimental non-alcoholic fatty liver disease models rely on the application of a diet or the administration of drugs to laboratory animals or the exposure of hepatic cell lines to these drugs. More recently, genetically modified rodents or zebrafish have been introduced as non-alcoholic fatty liver disease models. Considerable interest now lies in the discovery and development of novel non-invasive biomarkers of non-alcoholic fatty liver disease, with specific focus on hepatic steatosis. Experimental diagnostic biomarkers of non-alcoholic fatty liver disease, such as (epi)genetic parameters and '-omics'-based read-outs are still in their infancy, but show great promise. In this paper, the array of tools and models for the study of liver steatosis is discussed. Furthermore, the current state-of-art regarding experimental biomarkers such as epigenetic, genetic, transcriptomic, proteomic and metabonomic biomarkers will be reviewed.
Keywords: Biomarkers; Drugs; Models; Non-alcoholic fatty liver disease; Steatosis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
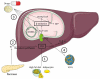
Figure 1. Pathogenesis of steatosis
Four pathogenic mechanisms can be responsible for the accumulation of TG-based lipid droplets, namely 1) increased uptake of FFAs from high-fat food or from adipocytes in body fat, 2) increased synthesis of FFAs in the liver from glucose or acetate by IR, 3) decreased mitochondrial β-oxidation of FFAs caused by a multitude of drugs, and 4) decreased synthesis or secretion of very low density lipoproteins, the principal route for elimination of lipids from the liver. (ATP, adenosine triphosphate; CoA, coenzyme A; FFA, free fatty acids; LDs, lipid droplets; IR, insulin resistance; TGs, triglyceride; VLDL, very low density lipoproteins).
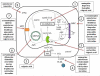
Figure 2. Mechanisms of drug-induced liver steatosis
Drugs may induce liver steatosis by a plethora of mechanisms associated with mitochondrial impairment, including 1) inhibition of enzymes involved in mitochondrial FFA oxidation leading to a direct alteration in β-oxidation function, 2) direct inhibition of the mitochondrial respiratory chain reaction or alteration of oxidative phosphorylation, 3) decreased uptake of acyl-CoA due to the inhibition of carnitine palmitoyl transferase 1, 4) reduction in triglyceride excretion by inhibition of microsomal triglyceride transfer protein, 5) impaired entrance of fatty acids in mitochondria due to the inhibition of acyl-CoA synthase, 6) depletion of mitochondrial DNA, encoding for mitochondrial proteins, which leads to impairment of MRC, 7) induction of the mitochondrial permeability transition pore opening, 8) alteration of mitochondrial membrane potential. (ACS, acyl-coenzyme A synthase; ADP, adenosine diphosphate; ATP, adenosine triphosphate; CoA, coenzyme A; CPT1, carnitine palmitoyl transferase 1; FAD, oxidized flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; FFA, free fatty acids; IMS, intermembrane space; MRC, mitochondrial respiratory chain; MPT, mitochondrial permeability transition; MTTP, mitochondrial triglyceride transfer protein; NAD, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; TG, triglyceride; VLDL, very low density lipoproteins).
Figure 3. Histological patterns of liver steatosis
Hematoxylin and eosin (upper panel) and Oil Red (lower panel) stained liver slice from C57BL/6 mice fed a high-fat choline-deficient diet for 8 weeks developing mixed macrovesicular and microvesicular steatosis. Both pictures depict the accumulation of TGs in the cytosol of hepatocytes. However, in case of macrovesicular steatosis, large lipid droplets are observed, which displace the cytoplasmic content and nucleus, while in microvesicular steatosis, small lipid droplets that do not displace the nucleus are seen (MA, macrovesicular steatosis; MI, microvesicular steatosis).
Similar articles
- Addition of trans fat and alcohol has divergent effects on atherogenic diet-induced liver injury in rodent models of steatohepatitis.
Daniels SJ, Leeming DJ, Detlefsen S, Bruun MF, Hjuler ST, Henriksen K, Hein P, Krag A, Karsdal MA, Nielsen MJ, Brockbank S, Cruwys S. Daniels SJ, et al. Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G410-G418. doi: 10.1152/ajpgi.00066.2019. Epub 2020 Jan 6. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31905026 - Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD.
Weber M, Mera P, Casas J, Salvador J, Rodríguez A, Alonso S, Sebastián D, Soler-Vázquez MC, Montironi C, Recalde S, Fucho R, Calderón-Domínguez M, Mir JF, Bartrons R, Escola-Gil JC, Sánchez-Infantes D, Zorzano A, Llorente-Cortes V, Casals N, Valentí V, Frühbeck G, Herrero L, Serra D. Weber M, et al. FASEB J. 2020 Sep;34(9):11816-11837. doi: 10.1096/fj.202000678R. Epub 2020 Jul 15. FASEB J. 2020. PMID: 32666604 - Biochemical and histological characterisation of an experimental rodent model of non-alcoholic steatohepatitis - Effects of a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist and a glucagon-like peptide-1 analogue.
Daniels SJ, Leeming DJ, Detlefsen S, Bruun MF, Hjuler ST, Henriksen K, Hein P, Karsdal MA, Brockbank S, Cruwys S. Daniels SJ, et al. Biomed Pharmacother. 2019 Mar;111:926-933. doi: 10.1016/j.biopha.2018.12.130. Epub 2019 Jan 7. Biomed Pharmacother. 2019. PMID: 30841472 - Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis.
Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Pejnovic N, et al. World J Gastroenterol. 2016 Nov 28;22(44):9706-9717. doi: 10.3748/wjg.v22.i44.9706. World J Gastroenterol. 2016. PMID: 27956794 Free PMC article. Review.
Cited by
- Targeting Mitochondrial ROS-Mediated Ferroptosis by Quercetin Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity.
Jiang JJ, Zhang GF, Zheng JY, Sun JH, Ding SB. Jiang JJ, et al. Front Pharmacol. 2022 Apr 12;13:876550. doi: 10.3389/fphar.2022.876550. eCollection 2022. Front Pharmacol. 2022. PMID: 35496312 Free PMC article. - A Comprehensive Study of High Cholesterol Diet-Induced Larval Zebrafish Model: A Short-Time In Vivo Screening Method for Non-Alcoholic Fatty Liver Disease Drugs.
Ma J, Yin H, Li M, Deng Y, Ahmad O, Qin G, He Q, Li J, Gao K, Zhu J, Wang B, Wu S, Wang T, Shang J. Ma J, et al. Int J Biol Sci. 2019 Mar 10;15(5):973-983. doi: 10.7150/ijbs.30013. eCollection 2019. Int J Biol Sci. 2019. PMID: 31182918 Free PMC article. - Mild Hypothermia Attenuates Hepatic Ischemia-Reperfusion Injury through Regulating the JAK2/STAT3-CPT1a-Dependent Fatty Acid _β_-Oxidation.
Wang W, Hu X, Xia Z, Liu Z, Zhong Z, Lu Z, Liu A, Ye S, Cao Q, Wang Y, Zhu F, Ye Q. Wang W, et al. Oxid Med Cell Longev. 2020 Mar 20;2020:5849794. doi: 10.1155/2020/5849794. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32256954 Free PMC article. - Fat content, fatty acid pattern and iron content in livers of turkeys with hepatic lipidosis.
Visscher C, Middendorf L, Günther R, Engels A, Leibfacher C, Möhle H, Düngelhoef K, Weier S, Haider W, Radko D. Visscher C, et al. Lipids Health Dis. 2017 May 30;16(1):98. doi: 10.1186/s12944-017-0484-8. Lipids Health Dis. 2017. PMID: 28558775 Free PMC article. - Topological and functional analysis of nonalcoholic steatohepatitis through protein interaction mapping.
Asadzadeh-Aghdaee H, Mansouri V, Peyvandi AA, Moztarzadeh F, Okhovatian F, Lahmi F, Vafaee R, Zali MR. Asadzadeh-Aghdaee H, et al. Gastroenterol Hepatol Bed Bench. 2016 Dec;9(Suppl1):S23-S28. Gastroenterol Hepatol Bed Bench. 2016. PMID: 28224024 Free PMC article.
References
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. - PubMed
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. - PubMed
- Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–62. - PubMed
- Bedogni G, Bellentani S. Fatty liver: how frequent is it and why? Ann Hepatol. 2004;3:63–5. - PubMed
- Bellentani S, Bedogni G, Miglioli L, Tiribelli C. The epidemiology of fatty liver. Eur J Gastroenterol Hepatol. 2004;16:1087–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical