Systemic LPS Translocation Activates Cross-Presenting Dendritic Cells but Is Dispensable for the Breakdown of CD8+ T Cell Peripheral Tolerance in Irradiated Mice - PubMed (original) (raw)
Systemic LPS Translocation Activates Cross-Presenting Dendritic Cells but Is Dispensable for the Breakdown of CD8+ T Cell Peripheral Tolerance in Irradiated Mice
Gabriel Espinosa-Carrasco et al. PLoS One. 2015.
Abstract
Lymphodepletion is currently used to enhance the efficacy of cytotoxic T lymphocyte adoptive transfer immunotherapy against cancer. This beneficial effect of conditioning regimens is due, at least in part, to promoting the breakdown of peripheral CD8+ T cell tolerance. Lymphodepletion by total body irradiation induces systemic translocation of commensal bacteria LPS from the gastrointestinal tract. Since LPS is a potent activator of the innate immune system, including antigen presenting dendritic cells, we hypothesized that LPS translocation could be required for the breakdown of peripheral tolerance observed in irradiated mice. To address this issue, we have treated irradiated mice with antibiotics in order to prevent LPS translocation and utilized them in T cell adoptive transfer experiments. Surprisingly, we found that despite of completely blocking LPS translocation into the bloodstream, antibiotic treatment did not prevent the breakdown of peripheral tolerance. Although irradiation induced the activation of cross-presenting CD8+ dendritic cells in the lymphoid tissue, LPS could not solely account for this effect. Activation of dendritic cells by mechanisms other than LPS translocation is sufficient to promote the differentiation of potentially autoreactive CD8+ T cells into effectors in irradiated mice. Our data indicate that LPS translocation is dispensable for the breakdown of CD8+ T cell tolerance in irradiated mice.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
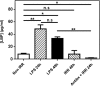
Fig 1. Depletion of immune cell populations in irradiated BALB/c mice.
BALB/c mice were sacrificed 24h after irradiation, non-irradiated mice served as controls, and T cells, NK cells, B cells, Monocyte/Macrophages and DCs were enumerated. Absolute numbers of CD3+ CD8+ T cells, CD3+ CD4+ T cells, CD3- DX5+ NK cells, CD19+ B cells, CD11b+ F4/80+ Mono/Macro, CD3- CD19- DX5- CD11c+ MHC II+ DC and CD3- CD19- DX5- CD11c+ MHC II+ CD8+ DC in the LN are represented as means ± SD (n = 4–8) from two independent experiments out of four.
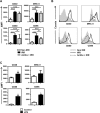
Fig 2. Irradiation-induced systemic LPS translocation is prevented by antibiotics in BALB/c mice.
Sera from BALB/c mice were collected 24h (IRR 24h) or 48h (IRR 48h) after irradiation. Antibiotic-treated BALB/c mice were irradiated 8 days later and sera collected 24h after irradiation (Antibx + IRR 24h). Sera collected 24h after Ultrapure LPS i.p. injection from non-irradiated BALB/c mice served as positive control (LPS 24h). Sera from non-irradiated mice served as negative control (Non IRR). Concentration of LBP in serum is presented as means ± SD (n = 4–7) from 3 independent experiments.
Fig 3. Antibiotics partially block irradiation-induced activation of CD11c+ DC.
(A) Non-irradiated, irradiated and antibiotic-treated irradiated groups of BALB/c mice have been described in Fig 2. Mice were sacrificed 24h after irradiation and the expression of CD40, MHC class II, CD80 and CD86 on gated living CD3- CD19- DX5- CD11c+ MHC II+ DC from the LN was analyzed by FACS. Increase in MFI respect to isotype-matched controls is represented as means ± SD (n = 6–8) from two independent experiments out of four. (B) Histograms represent the phenotype of CD3- CD19- DX5- CD11c+ MHC II+ DC from individual representative mice described in panel A. (C) Non-irradiated and LPS-injected groups of mice described in Fig 2 were sacrificed 24 after treatment. Expression of CD40, MHC class II, CD80 and CD86 on gated living CD3- CD19- DX5- CD11c+ MHC II+ DC was analyzed by FACS. Increase in MFI respect to isotype-matched controls is represented as means ± SD (n = 3–4) from two independent experiments out of three.
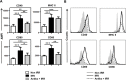
Fig 4. Antibiotics partially block irradiation-induced activation of CD8+ DC.
(A) Non-irradiated, irradiated and antibiotic-treated irradiated groups of BALB/c mice have been described in Fig 2. Mice were sacrificed 24h after irradiation and the expression of CD40, MHC class II, CD80 and CD86 on gated living CD3- CD19- DX5- CD11c+ MHC II+ CD8+ DC from the LN was analyzed by FACS. Increase in MFI respect to isotype-matched controls is represented as means ± SD (n = 6–8) from two independent experiments out of four. (B) Histograms represent the phenotype of CD3- CD19- DX5- CD11c+ MHC II+ CD8+ DC from individual representative mice described in panel A.
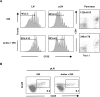
Fig 5. Antibiotics do not prevent antigen-driven proliferation and differentiation of Clone 4 CD8+ T cells into effectors in irradiated InsHA mice.
(A) Irradiated and antibiotic-treated irradiated groups of InsHA mice adoptively transferred with 5x106 CFSE-labeled naïve Clone 4 Thy1.1+ CD8+ and 5x106 HNT CD4+ T cells were sacrificed on day 10 after transfer. CFSE fluorescence intensity on gated CD8+ Thy1.1+ donor lymphocytes in LN and pLN of single representative mice are shown in left and central panels. Percentages of highly proliferating cells, means ± SD (n = 4), of one experiment out three are presented. The presence of CD8+ Thy1.1+ donor T cells in the pancreas is shown in right panels. Numbers indicate FACS event counts in the depicted gates as means ± SD (n = 4) of one representative experiment out three. (B) Phenotype of CD8+ Thy1.1+ donor lymphocytes in the pLN of individual InsHA mice from groups described in panel A. Percentages of CD25+ Granzyme B+ of the donor T cells from pooled pLN of one experiment out of two.
Similar articles
- A CD40/CD40L feedback loop drives the breakdown of CD8(+) T-cell tolerance following depletion of suppressive CD4(+) T cells.
Muth S, Schütze K, Hain T, Yagita H, Schild H, Probst HC. Muth S, et al. Eur J Immunol. 2014 Apr;44(4):1099-107. doi: 10.1002/eji.201343738. Epub 2014 Jan 20. Eur J Immunol. 2014. PMID: 24420080 - Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling.
Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Paulos CM, et al. J Clin Invest. 2007 Aug;117(8):2197-204. doi: 10.1172/JCI32205. J Clin Invest. 2007. PMID: 17657310 Free PMC article. - Antigens expressed by myelinating glia cells induce peripheral cross-tolerance of endogenous CD8+ T cells.
Schildknecht A, Probst HC, McCoy KD, Miescher I, Brenner C, Leone DP, Suter U, Ohashi PS, van den Broek M. Schildknecht A, et al. Eur J Immunol. 2009 Jun;39(6):1505-15. doi: 10.1002/eji.200839019. Eur J Immunol. 2009. PMID: 19462379 - Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells.
Lutz MB, Kurts C. Lutz MB, et al. Eur J Immunol. 2009 Sep;39(9):2325-30. doi: 10.1002/eji.200939548. Eur J Immunol. 2009. PMID: 19701895 Review. - [Dendritic cells of mucosa and skin: "recruited for vaccination"].
Le Borgne M, Dubois B, Kaiserlian D. Le Borgne M, et al. Med Sci (Paris). 2007 Oct;23(10):819-25. doi: 10.1051/medsci/20072310819. Med Sci (Paris). 2007. PMID: 17937889 Review. French.
Cited by
- The role of mitochondrial transfer in the suppression of CD8+ T cell responses by Mesenchymal stem cells.
Vaillant L, Akhter W, Nakhle J, Simon M, Villalba M, Jorgensen C, Vignais ML, Hernandez J. Vaillant L, et al. Stem Cell Res Ther. 2024 Nov 4;15(1):394. doi: 10.1186/s13287-024-03980-1. Stem Cell Res Ther. 2024. PMID: 39497203 Free PMC article. - Transfer of mesenchymal stem cell mitochondria to CD4+ T cells contributes to repress Th1 differentiation by downregulating T-bet expression.
Akhter W, Nakhle J, Vaillant L, Garcin G, Le Saout C, Simon M, Crozet C, Djouad F, Jorgensen C, Vignais ML, Hernandez J. Akhter W, et al. Stem Cell Res Ther. 2023 Jan 24;14(1):12. doi: 10.1186/s13287-022-03219-x. Stem Cell Res Ther. 2023. PMID: 36694226 Free PMC article. - The emerging roles of the gut microbiome in allogeneic hematopoietic stem cell transplantation.
Khuat LT, Dave M, Murphy WJ. Khuat LT, et al. Gut Microbes. 2021 Jan-Dec;13(1):1966262. doi: 10.1080/19490976.2021.1966262. Gut Microbes. 2021. PMID: 34455917 Free PMC article. Review.
References
- Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6(3):205–14. - PubMed
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006. December;12(12):1365–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by grants from the European Community SUDOE-FEDER (Contract # IMMUNONET SOE1/P1/E014) and La Ligue Contre le Cancer Hérault to JH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials