Multivalency governs HP1α association dynamics with the silent chromatin state - PubMed (original) (raw)
Multivalency governs HP1α association dynamics with the silent chromatin state
Sinan Kilic et al. Nat Commun. 2015.
Abstract
Multivalent interactions between effector proteins and histone post-translational modifications are an elementary mechanism of dynamic chromatin signalling. Here we elucidate the mechanism how heterochromatin protein 1α (HP1α), a multivalent effector, is efficiently recruited to the silent chromatin state (marked by trimethylated H3 at Lys9, H3K9me3) while remaining highly dynamic. Employing chemically defined nucleosome arrays together with single-molecule total internal reflection fluorescence microscopy (smTIRFM), we demonstrate that the HP1α residence time on chromatin depends on the density of H3K9me3, as dissociated factors can rapidly rebind at neighbouring sites. Moreover, by chemically controlling HP1α dimerization we find that effector multivalency prolongs chromatin retention and, importantly, accelerates the association rate. This effect results from increased avidity together with strengthened nonspecific chromatin interactions of dimeric HP1α. We propose that accelerated chromatin binding is a key feature of effector multivalency, allowing for fast and efficient competition for binding sites in the crowded nuclear compartment.
Figures
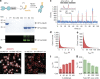
Figure 1. An assay to measure effector interaction dynamics with chromatin arrays.
(a) Scheme of the experiment: Atto532-labelled HP1α dynamically interacts with immobilized H3K9me3-modified designer chromatin arrays, while the dynamic interactions are detected by smTIRFM. (b) Synthesis of H3K9me3 using a traceless EPL approach: (i) Oxidation of the hydrazide, in situ thioester formation and EPL reaction, (ii) desulfurization. Left: RP-HPLC analysis of the final protein, right: ESI-MS analysis of the final histone (MW: 15,251.8 Da calculated, 15,252 Da observed). (c) Design of chromatin DNA: recombinantly produced DNA carrying twelve 601-nucleosome position sequences (NPS) separated by 30 bp linkers, and a single, unique restriction site is ligated to a synthetic biotinylated and Atto647N-labelled oligonucleotide. (d) Chromatin array immobilization is specific and depends on neutravidin anchoring (−NA, neutravidin absent, +NA, neutravidin present) as observed by Atto647N fluorescence emission. Scale bars, 5 μm. (e) Atto488-labelled H2A, incorporated into the arrays, co-localizes with Atto647N-labelled DNA (orange spots).
Figure 2. HP1α dynamically interacts with chromatin fibres.
(a) HP1α fused to NpuN is expressed, bound to NpuC-beads, cleaved in ligation buffer and reacted with P1 in situ, yielding labelled HP1α (HP1α(A532)). (I=input; FT=flowthrough; W1-3=washes; E1-3=elutions). (b) Left: single chromatin arrays are detected by Atto647N emission. Right: HP1α interaction dynamics are monitored by Atto532 emission. Scale bar, 5 μm. (c) Time trace of fluorescence intensity (blue) from a single chromatin array showing transient HP1α binding events, fitted by a step function (red). Inset: determination of bound (on-) and unbound (off-) times by a thresholding algorithm. (d) Dissociation kinetics: cumulative histogram of binding intervals (_t_bright) for 100 chromatin arrays (105 frames each), fitted by a double-exponential function (fit: _τ_off,1=0.25±0.03 s, _τ_off,2=2.26±1.22 s). (e) Association kinetics: cumulative histogram of intervals between binding events (_t_dark) over 30 chromatin arrays, fitted by a single-exponential function (fit: _λ_on=22.9±9.8 s). (f) The HP1α residence time (_τ_off,1) depends on H3K9me3 density. Numbers indicate % amplitude of the fast phase (errors: s.e.m.; n =2–16 replicates). (g) The HP1α residence time (_τ_off,1) decreases as a function of HP1α concentration due to local competition. The indicated concentration of unlabelled HP1α is added to 1 nM Atto532-labelled HP1α. Numbers indicate % amplitude of the fast phase (errors: s.e.m., _n_=3–16 replicates). (d–g) For all fit results, see Table 1.
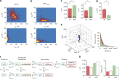
Figure 3. Affinity-directed HP1α dimerization allows investigating multivalent chromatin binding.
(a) Strategy to synthesize HP1αcdm employing the PxVxI peptide P2 in an affinity-directed dual-EPL reaction (PDB: 3Q6S, ref. 39). (b) One-pot production of Atto532-labelled HP1αcdm: HP1α(1–177), fused to the NpuN-split intein was bound to NpuC-beads and eluted with ligation buffer containing the hSgoL1 peptide (carrying two cysteinyl-lysines and an Atto532 dye), yielding labelled HP1αcdm (Atto532). Pure HP1αcmd was obtained after gel-filtration purification. Gel annotation: I=input; FT=flowthrough of column; W1-2=column washes; E1-3=elution fractions; Fn=final protein after gel filtration. (c) Time trace of fluorescence intensity (blue) from a single chromatin array fitted by a step function (red) and showing transient HP1αcdm binding events (at 0.5 nM). (d) HP1αcdm dissociation kinetics (100 chromatin arrays, 105 frames each) fitted by a double-exponential function (fit: _τ_off,1=0.33±0.01 s, _τ_off,2=3.40±0.53 s). (e) HP1αcdm association kinetics over 30 chromatin arrays, fitted by a single-exponential function (fit: _λ_on=7.45±1.87 s). (d,e) For all fit results, see Table 1.
Figure 4. Multivalent HP1α-chromatin interactions increase association speed.
(a) Distribution of interaction kinetics from HP1α with single chromatin arrays: Two dimensional histograms of single-array binding kinetics showing the correlation of dissociation time constants, _τ_off,1 and _τ_off,2 with the apparent association time constant _λ_on. (b) Two dimensional histogram of single-array binding kinetics for HP1αcdm. (c) Comparison of the average dissociation time constants _τ_off,1 and _τ_off,2 (fast and slow phase) between HP1α and HP1αcdm (errors: s.e.m., _n_=4–16 replicates, *P<0.05, Student's _t_-test). (d) Comparison of the association rate constant k on between HP1α and HP1αcdm (errors: s.e.m., _n_=4–16 replicates, *P<0.05, Student's _t_-test). (c,d) For all fit results, see Table 1. (e) Brownian dynamics simulation of chromatin binding: monomeric or dimeric binders are diffusing in a box with 200 nm edge length containing a 12-nucleosome chromatin array. First passage binding kinetics are plotted for monomeric (red) and dimeric (green) binders at 20 and 10 μM binder concentration, respectively. Single-exponential fits yield _λ_on=53.6 _τ_D for the dimeric and _λ_on=89 _τ_D for the monomeric binder (_τ_D is the characteristic diffusion time, τ_D_=6πηa 3 /(k_B_T) for binders with radius a in solvent with viscosity η, k_B_T being the thermal energy). (f) Kinetic model for chromatin binding of HP1α: HP1α in a monomer-dimer equilibrium (_k_mono, _k_dim) associates nonspecifically (_k_assoc) and reversibly (_k_dissoc) with nucleosomes (short-lived interactions with (i) the nucleosomal or linker DNA, (ii) the nucleosomal surface or (iii) different regions on histone tails) before transitioning to a specific, H3K9me3 bound state (_k_bind) with a 100 ms-residence time (_k_release). The nonspecific association of dimeric protein is prolonged by a factor α, and it can further proceed to a bivalent state (_k_biv). (g) Comparison of experimental data of HP1α and HP1αcdm chromatin binding with results from kinetic model in (f). For parameters, see Supplementary Table 1.
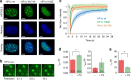
Figure 5. CSD interactions accelerate HP1 association dynamics.
(a) Confocal flurescence images of NIH 3T3 cells transfected with mEos3.2-HP1a, mEos3.2-HP1α(I163E) or mEos3.2-HP1α(W174A) and overlay with Hoechst staining. Scale bar, 5 μm. (b) Time series of images from a typical FRAP experiment (HP1α), showing the bleached spot as a red circle pre- and post bleach at indicated times. Scale bar, 5 μm. (c) FRAP analysis of mEos3.2-HP1α wt or W174A in heterochromatin foci (_n_=15 cells with two foci from each, the shaded area denotes the standard deviation at each time point). Analysis of the traces using a diffusion/binding model results in the following values: HP1α: _λ_on=1.2±0.4 s and _τ_off=2.8±1.2 s, HP1α(W174A): _λ_on=1.0±0.2 s and _τ_off=1.1±0.3 s, HP1α(I163E): _λ_on=1.8±0.3 s and _τ_off=0.8±0.1 s. (c) Comparison of the average dissociation time constants _τ_off,1 and _τ_off,2 (fast and slow phase) between HP1α in the absence and presence of P3 (error bars: s.e.m., _n_=4–16 replicates, *P<0.05, Student's _t_-test). (d) Comparison of the average dissociation time constants _τ_off,1 and _τ_off,2 (fast and slow phase) between HP1α in the absence and presence of P3 (error bars: s.e.m., _n_=4–16 replicates, *P<0.05, Student's _t_-test). (e) Comparison of the association rate constant _k_on between HP1α in the absence and presence of P3 (errors: s.e.m., _n_=4–16 replicates, *P<0.05, Student's _t_-test). (c,d) For all fit results, see Table 1.
Similar articles
- Single-molecule kinetic analysis of HP1-chromatin binding reveals a dynamic network of histone modification and DNA interactions.
Bryan LC, Weilandt DR, Bachmann AL, Kilic S, Lechner CC, Odermatt PD, Fantner GE, Georgeon S, Hantschel O, Hatzimanikatis V, Fierz B. Bryan LC, et al. Nucleic Acids Res. 2017 Oct 13;45(18):10504-10517. doi: 10.1093/nar/gkx697. Nucleic Acids Res. 2017. PMID: 28985346 Free PMC article. - The interplay between H2A.Z and H3K9 methylation in regulating HP1α binding to linker histone-containing chromatin.
Ryan DP, Tremethick DJ. Ryan DP, et al. Nucleic Acids Res. 2018 Oct 12;46(18):9353-9366. doi: 10.1093/nar/gky632. Nucleic Acids Res. 2018. PMID: 30007360 Free PMC article. - Polycomb repressive complex 2 and H3K27me3 cooperate with H3K9 methylation to maintain heterochromatin protein 1α at chromatin.
Boros J, Arnoult N, Stroobant V, Collet JF, Decottignies A. Boros J, et al. Mol Cell Biol. 2014 Oct 1;34(19):3662-74. doi: 10.1128/MCB.00205-14. Epub 2014 Jul 21. Mol Cell Biol. 2014. PMID: 25047840 Free PMC article. - Structure and function of protein modules in chromatin biology.
Yap KL, Zhou MM. Yap KL, et al. Results Probl Cell Differ. 2006;41:1-23. Results Probl Cell Differ. 2006. PMID: 16909888 Review. - Histone variants: key players of chromatin.
Biterge B, Schneider R. Biterge B, et al. Cell Tissue Res. 2014 Jun;356(3):457-66. doi: 10.1007/s00441-014-1862-4. Epub 2014 Apr 30. Cell Tissue Res. 2014. PMID: 24781148 Review.
Cited by
- Interactions of HP1 Bound to H3K9me3 Dinucleosome by Molecular Simulations and Biochemical Assays.
Watanabe S, Mishima Y, Shimizu M, Suetake I, Takada S. Watanabe S, et al. Biophys J. 2018 May 22;114(10):2336-2351. doi: 10.1016/j.bpj.2018.03.025. Epub 2018 Apr 21. Biophys J. 2018. PMID: 29685391 Free PMC article. - Nucleosome Dynamics Derived at the Single-Molecule Level Bridges Its Structures and Functions.
Chen P, Li G, Li W. Chen P, et al. JACS Au. 2024 Feb 26;4(3):866-876. doi: 10.1021/jacsau.3c00658. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559720 Free PMC article. Review. - Interplay between epigenome and 3D chromatin structure.
Han MH, Issagulova D, Park M. Han MH, et al. BMB Rep. 2023 Dec;56(12):633-644. doi: 10.5483/BMBRep.2023-0197. BMB Rep. 2023. PMID: 38052424 Free PMC article. Review. - Mechanisms of noncanonical binding dynamics in multivalent protein-protein interactions.
Errington WJ, Bruncsics B, Sarkar CA. Errington WJ, et al. Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25659-25667. doi: 10.1073/pnas.1902909116. Epub 2019 Nov 27. Proc Natl Acad Sci U S A. 2019. PMID: 31776263 Free PMC article. - Characterization of Hepatoma-Derived Growth Factor-Related Protein 2 Interactions with Heterochromatin.
Wistner SC, MacDonald IA, Stanley KA, Hathaway NA. Wistner SC, et al. Cells. 2023 Jan 14;12(2):325. doi: 10.3390/cells12020325. Cells. 2023. PMID: 36672260 Free PMC article.
References
- Kouzarides T. Chromatin modifications and their function. Cell 128, 693–705 (2007) . - PubMed
- Hediger F. & Gasser S. M. Heterochromatin protein 1: don't judge the book by its cover!. Curr. Opin. Genet. Dev. 16, 143–150 (2006) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources