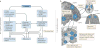The neuroscience of placebo effects: connecting context, learning and health - PubMed (original) (raw)
Review
The neuroscience of placebo effects: connecting context, learning and health
Tor D Wager et al. Nat Rev Neurosci. 2015 Jul.
Abstract
Placebo effects are beneficial effects that are attributable to the brain-mind responses to the context in which a treatment is delivered rather than to the specific actions of the drug. They are mediated by diverse processes--including learning, expectations and social cognition--and can influence various clinical and physiological outcomes related to health. Emerging neuroscience evidence implicates multiple brain systems and neurochemical mediators, including opioids and dopamine. We present an empirical review of the brain systems that are involved in placebo effects, focusing on placebo analgesia, and a conceptual framework linking these findings to the mind-brain processes that mediate them. This framework suggests that the neuropsychological processes that mediate placebo effects may be crucial for a wide array of therapeutic approaches, including many drugs.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
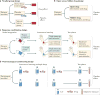
Figure 1. Elements of treatment context
Whether treatment consists of an active drug or a placebo, the clinical setting that surrounds treatment includes multiple types of context information that are perceived and interpreted by the patient’s brain. The external context includes treatment, place and social cues, along with verbal suggestions. The internal context consists of memories, emotions, expectancies and appraisals of the meaning of the context for future survival and well-being. These features combine to make up the treatment context and are the ‘active ingredients’ of placebo effects.
Figure 2. Paradigms for assessing placebo effects
Most paradigms used to assess placebo treatments fall into one of four categories. a | In a parallel group design, placebo effects are measured by comparing outcomes in a placebo group with those in a no-treatment control group. This is the most common paradigm in clinical trials. b | In an open versus hidden design, drugs are delivered either with (open) or without (hidden) the knowledge of the patient. This design permits assessment of the effects of treatment context in clinical settings without withholding treatment. Extended designs such as the balanced placebo design cross open versus hidden administration with verum versus sham drugs, enabling researchers to assess placebo–drug interactions. c | Response conditioning designs use instructions combined with reinforcement to maximize the effectiveness of placebo treatments. In a common variant, initial verbal instructions are provided that one cream (the placebo) is an effective analgesic and another (the control) is not. Then, painful stimulation is given on both placebo-treated and control-treated skin sites. Participants are told that the stimulus intensity will be the same on both sites, but in fact it is surreptitiously reduced for the placebo-treated site, reinforcing belief in the placebo and associations with relief. During a final test phase, equivalent levels of painful stimulation are applied to both sites, and the effects of the placebo conditioning procedure are assessed. This is the most common paradigm used in neuroimaging studies; placebo and control treatments are often compared in a within-person crossover design. d | Pharmacological conditioning designs combine instructions and cues paired with active drugs during a conditioning phase, which often occurs over multiple days. Placebo effects are determined by presenting cues alone and comparing outcomes in drug-paired versus non-drug-paired groups. Response conditioning and pharmacological conditioning designs have been used in both humans and non-human animals.
Figure 3. The neurophysiology of placebo analgesia
a | An overview of the brain regions involved in the placebo effects on pain and their potential functions in this context. The areas shown in blue respond to painful stimuli and, on that basis, are expected to show reduced responses to pain after placebo treatment. These areas include the medial thalamus (mThal), anterior insula (aINS), dorsal anterior cingulate cortex (dACC), periaqueductal grey (PAG) and secondary somatosensory cortex–dorsal posterior insula (S2–dpINS). Areas shown in red are associated with increases in response to placebo treatment (either before or during painful stimulation), and activity in these regions is thought to be involved with the maintenance of context information and the generation of placebo-related expectations and appraisals. They include the ventromedial prefrontal cortex (vmPFC), dorsolateral PFC (dlPFC), lateral orbitofrontal cortex (lOFC), nucleus accumbens–ventral striatum (NAc–VS), PAG and rostroventral medulla (RVM). Some regions, including the PAG and dACC, show different effects depending on the study and timing relative to painful stimulation. b | Results from neuroimaging studies of placebo-induced analgesia. Each point represents a finding from an individual study, reported in standard Montreal Neurological Institute space (all studies are listed in Supplementary information S2 (box)). Red points show increases in activity under placebo versus control treatment (that is, the same cream without the belief that it is a painkiller), and blue points identify decreases in activity under placebo. These comparisons involved randomized assignment to placebo or control conditions, and so they can test the causal effects of placebo treatment on brain activity. Some studies also examined correlations between the magnitude of placebo analgesia and the magnitude of placebo-induced changes in brain responses. Orange points identify positive correlations between the magnitude of an individual’s activity increases under placebo versus control treatment and the magnitude of placebo analgesia. Light blue points identify negative correlations. These correlations do not necessarily reflect causal effects of placebo on brain activity but can provide important information on the nature of the individual differences that predispose a person towards showing a larger versus a smaller placebo response.
Figure 4. Concepts, associations and the representation of context
a | Patient outcomes, and hence placebo effects, are measured as a function of pathophysiology (signs), reported experiences (symptoms) and behaviour. These outcomes are influenced in various ways by the two primary components of the treatment context: conceptual processes and pre-cognitive associations. Conceptual processes can influence expectations, appraisals and memories, which can directly influence emotional states, reported decisions and behaviour. Pre-cognitive associations influence physiological processes outside conscious control, which can in turn influence emotion, motivation and affective states as well as outcome measures. Thus, some types of placebo effects may be mediated by affective and motivational states, whereas others may be independent of such states, depending on the nature of the context and the outcome. b | Conceptual processes have been difficult to define and measure precisely in the brain, because they depend on the integration of information associated with multiple systems into an overall schema, or conceptualization of the situation and its implications for well-being, which guides the meaning or significance of events. The ingredients of such ‘meaning responses’, which are thought to be critical for placebo effects, include inferences about social information (dorsomedial prefrontal cortex (dmPFC)), interoceptive assessments of one’s body state (insula), expectancies (lateral orbitofrontal cortex (lOFC)) and autobiographical memories and place context information (hippocampus (Hipp)). The ventromedial PFC (vmPFC) is positioned to integrate these elements into a coherent schema that informs and is informed by responses at other processing levels, including brainstem and subcortical centres that regulate sensory, autonomic and neuroendocrine responses. AMY, amygdala; HYP, hypothalamus; NAc, nucleus accumbens; PAG, periaqueductal grey; RVM, rostroventral medulla.
Similar articles
- The Cognitive Neuroscience of Placebo Effects: Concepts, Predictions, and Physiology.
Geuter S, Koban L, Wager TD. Geuter S, et al. Annu Rev Neurosci. 2017 Jul 25;40:167-188. doi: 10.1146/annurev-neuro-072116-031132. Epub 2017 Apr 7. Annu Rev Neurosci. 2017. PMID: 28399689 Review. - Placebo responders and nonresponders: what's new?
Frisaldi E, Shaibani A, Benedetti F. Frisaldi E, et al. Pain Manag. 2018 Nov 1;8(6):405-408. doi: 10.2217/pmt-2018-0054. Pain Manag. 2018. PMID: 30873895 No abstract available. - Placebo analgesia and beyond: a melting pot of concepts and ideas for neuroscience.
Carlino E, Pollo A, Benedetti F. Carlino E, et al. Curr Opin Anaesthesiol. 2011 Oct;24(5):540-4. doi: 10.1097/ACO.0b013e328349d0c2. Curr Opin Anaesthesiol. 2011. PMID: 21772145 Review. - [Neurobiological and neurochemical mechanisms of placebo analgesia].
Asan L, Bingel U, Kunkel A. Asan L, et al. Schmerz. 2022 Jun;36(3):205-212. doi: 10.1007/s00482-022-00630-4. Epub 2022 Mar 17. Schmerz. 2022. PMID: 35301592 Free PMC article. Review. German. - How placebos change the patient's brain.
Benedetti F, Carlino E, Pollo A. Benedetti F, et al. Neuropsychopharmacology. 2011 Jan;36(1):339-54. doi: 10.1038/npp.2010.81. Epub 2010 Jun 30. Neuropsychopharmacology. 2011. PMID: 20592717 Free PMC article. Review.
Cited by
- Decoding the impact of the placebo response in clinical trials for chronic cough.
Zhang M, Zhang B, Morice AH. Zhang M, et al. ERJ Open Res. 2024 Oct 28;10(5):00335-2024. doi: 10.1183/23120541.00335-2024. eCollection 2024 Sep. ERJ Open Res. 2024. PMID: 39469270 Free PMC article. - Pre-Molecular Assessment of Self-Processes in Neurotypical Subjects Using a Single Cognitive Behavioral Intervention Evoking Autobiographical Memory.
Martins JE, Simões J, Barros M, Simões M. Martins JE, et al. Behav Sci (Basel). 2022 Oct 5;12(10):381. doi: 10.3390/bs12100381. Behav Sci (Basel). 2022. PMID: 36285950 Free PMC article. - Placebo stimulates neuroplasticity in depression: implications for clinical practice and research.
Seymour J, Mathers N. Seymour J, et al. Front Psychiatry. 2024 Jan 10;14:1301143. doi: 10.3389/fpsyt.2023.1301143. eCollection 2023. Front Psychiatry. 2024. PMID: 38268561 Free PMC article. - The Brain from Within.
di Porzio U. di Porzio U. Front Hum Neurosci. 2016 Jun 8;10:265. doi: 10.3389/fnhum.2016.00265. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27375460 Free PMC article. Review. - An externally validated resting-state brain connectivity signature of pain-related learning.
Kincses B, Forkmann K, Schlitt F, Jan Pawlik R, Schmidt K, Timmann D, Elsenbruch S, Wiech K, Bingel U, Spisak T. Kincses B, et al. Commun Biol. 2024 Jul 17;7(1):875. doi: 10.1038/s42003-024-06574-y. Commun Biol. 2024. PMID: 39020002 Free PMC article.
References
- Institute of Medicine of the National Academies, editor. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; 2011. pp. 1–350. - PubMed
- Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84:623–637. This review discusses the pharmacological foundation of many types of placebo effects and addresses the translational and ethical implications of placebo studies. - PubMed
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. - PubMed
- Meissner K. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Phil. TransRSoc. B. 2011;366:1808–1817. This review focuses on the evidence that placebos influence autonomic nervous system responses, including effects on gastrointestinal, cardiovascular and pulmonary functions. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DA027794/DA/NIDA NIH HHS/United States
- Intramural NIH HHS/United States
- R01DA027794/DA/NIDA NIH HHS/United States
- 2R01MH076136/MH/NIMH NIH HHS/United States
- R01 MH076136/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources