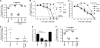VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells - PubMed (original) (raw)
. 2015 Jun 19;348(6241):aaa8205.
doi: 10.1126/science.aaa8205.
Andrew Olive # 1, Aleksandar F Radovic-Moreno # 2 3, David Gondek 1, David Alvarez 1, Pamela A Basto 2 3, Mario Perro 1, Vladimir D Vrbanac 4, Andrew M Tager 4, Jinjun Shi 5, Jeremy A Yethon 6, Omid C Farokhzad 5 7, Robert Langer 2 3, Michael N Starnbach 1, Ulrich H von Andrian 1 8
Affiliations
- PMID: 26089520
- PMCID: PMC4605428
- DOI: 10.1126/science.aaa8205
VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells
Georg Stary et al. Science. 2015.
Abstract
Genital Chlamydia trachomatis (Ct) infection induces protective immunity that depends on interferon-γ-producing CD4 T cells. By contrast, we report that mucosal exposure to ultraviolet light (UV)-inactivated Ct (UV-Ct) generated regulatory T cells that exacerbated subsequent Ct infection. We show that mucosal immunization with UV-Ct complexed with charge-switching synthetic adjuvant particles (cSAPs) elicited long-lived protection in conventional and humanized mice. UV-Ct-cSAP targeted immunogenic uterine CD11b(+)CD103(-) dendritic cells (DCs), whereas UV-Ct accumulated in tolerogenic CD11b(-)CD103(+) DCs. Regardless of vaccination route, UV-Ct-cSAP induced systemic memory T cells, but only mucosal vaccination induced effector T cells that rapidly seeded uterine mucosa with resident memory T cells (T(RM) cells). Optimal Ct clearance required both T(RM) seeding and subsequent infection-induced recruitment of circulating memory T cells. Thus, UV-Ct-cSAP vaccination generated two synergistic memory T cell subsets with distinct migratory properties.
Copyright © 2015, American Association for the Advancement of Science.
Figures
Figure 1. Differential effects of immunization with Ct / UV-Ct and conjugation of UV-Ct with synthetic nanoparticles
(A) Schematic diagram of the immunization and challenge protocol for Figs. 1B-C, 2A-C, 2F, 3A-B, 4E-F, 6A, S4 and S7. Mice were immunized with Ct, UV-Ct, UV-_Ct_-cSAP or UV-Ct mixed with control nanoparticles (Ct, live Chlamydia trachomatis; UV-Ct, inactivated Chlamydia trachomatis; UV-_Ct_-cSAP, inactivated Chlamydia trachomatis complexed with charge-switching synthetic adjuvant particles; UV-Ct + SAP, inactivated Chlamydia trachomatis mixed with synthetic adjuvant particles (not attached to UV-Ct); UV-_Ct_-cSP inactivated Chlamydia trachomatis complexed with charge-switching synthetic particles (without adjuvant)) via intrauterine (i.u.), intranasal (i.n.) or subcutaneous (s.c.) routes. Challenge with live Ct was always i.u. (B) Uterine Ct burden was measured by qPCR 6 days post i.u. challenge with live Ct in naïve mice and in animals that had been immunized 4 weeks earlier by i.u. injection of infectious Ct or UV-Ct. Data are pooled from 4 independent experiments (n= 20 mice per group; **P<0.01; ***P<0.001; one-way ANOVA followed by Bonferroni's post-test). (C) Ct burden following i.u. or s.c. immunization with UV-Ct mixed with adjuvants: alum, aluminum hydroxide; IMQ, imiquimod; CpG, CpG oligodeoxynucleotide type C (n= 5-7 mice per group; **P<0.002; ***P<0.001; one-way ANOVA followed by Bonferroni's post-test). (D) Schematic representation of surface charge-switching synthetic adjuvant particle (cSAP) production and conjugation to UV-Ct. (E-F) UV-Ct stained with BacLight was incubated with Alexa Fluor488 labeled cSAP or SAP at pH 7.4 or 6.0. (E) Representative FACS plots and (F) quantification of nanoparticle conjugates with UV-Ct from 2 independent experiments. ***P<0.001; ns, not significant; two-tailed t-tests. (G) A representative cryo-transmission electron micrograph of a UV-_Ct_–cSAP cluster showing cSAP in red and UV-Ct in blue. Scale bar: 100 nm. (H) Dynamic light scattering profiles of UV-_Ct_–cSAP, cSAP and UV-Ct alone. The population distribution is representative of the volume scattering intensity. Data are representative of 10 independent experiments. Error bars represent mean ± SEM.
Figure 2. Intrauterine immunization with UV-_Ct_–cSAP protects against challenge with live Ct
(A) Ct burden following i.u. challenge with live Ct 4 weeks after immunization (n=4-10 mice/group; **P<0.01; ***P<0.001). (B-C) Timecourse of Ct burden following i.u. Ct challenge 4 weeks after immunization (n=4 mice/group/timepoint, ***P<0.001) measured by (B) qPCR or (C) in vitro assessment of inclusion-forming units (IFUs). (D) Ct burden following intravaginal challenge with Cm 4 weeks after immunization with Cm and UV-_Cm_-cSAP. n = 3-4 mice/group; *** P<0.001. (E) Gross uterine pathology determined as hydrosalpinx score 4 weeks after intravaginal challenge of immunized and naïve mice with Cm (n=8 mice/group; **P<0.01, ***P<0.001. (F) Ct burden following i.u. Ct challenge 6 months after i.u. im munization (n=4-10 mice/group; ***P<0.001). Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
Figure 3. Intrauterine immunization with UV-Ct or UV-_Ct_–cSAP leads to differential activation of _Ct_-specific CD4+ T cells
(A) Ct burden following i.u. Ct challenge of MHC-II−/−, CD8−/− or μMt mice 4 weeks after immunization (n=2-11 mice/group; ***P<0.001). (B) Ct burden in i.u. infected mice that received adoptive transfers of leukocyte subsets from naive or immunized donors. Pooled data from 2 independent experiments (n=4-7 mice/group; ***P<0.001). (C-F) Flow cytometric analysis of _Ct_-specific NR1 cells in uterus and draining iliac LNs 4 days after i.u. immunization. (C) NR1 cell proliferation in uterus draining LNs. Representative histograms show CFSE dilution, a measure of T cell proliferation, in one of three independent experiments. (D-E) Absolute number of NR1 cells recovered from (D) iliac LNs and (E) the uterus. (F) Intracellular cytokine staining for TNF-α, IFN-γ, and IL-2 in iliac LN-derived NR1 cells after ex vivo restimulation with Ag-pulsed DCs. Data are shown as percentage of total NR1 cells expressing each combination of cytokines (n=5 mice/group; *P<0.05). Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
Figure 4. UV-Ct-induced tolerance is mediated by FoxP3+ NR-1 cells
(A-D) Sorted GFP− NR1xFoxP3-eGFP TN were adoptively transferred to naïve mice prior to immunization. Total GFP+CD25+ NR1 cells (CD4+Vβ8.3+Vα2+) were enumerated by FACS in single-cell suspensions of (A, B) iliac LNs and (C, D) uterus 4 days after immunization and are shown as (A, C) representative contour plots of NR1 cells and (B, D) percent of total NR1 cells (n=5 mice/group; **P<0.01). (E) Ct burden following i.u. Ct challenge 4 weeks after immunization with Ct, UV-Ct or UV-_Ct_–cSAP. In some animals Treg were depleted with anti-CD25 mAb (clone PC61), while the other groups received isotype-matched IgG three days before and after challenge (n=6 mice/group; ***P<0.001). (F) Ct burden following i.u. challenge with live Ct 4 weeks after immunization of Il10−/− mice. (n=5-11 mice/group; *P<0.05; **P<0.01; ***P<0.001). Error bars depict mean ± SEM. Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
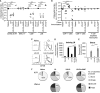
Figure 5. Distinct uterine DC subsets acquire Ags after i.u. Ct and UV-_Ct_-cSAP versus UV-Ct immunization and induce differential responses by _Ct_-specific T cells in vitro and in vivo
(A) Schematic diagram of the experimental protocol for panels B-F. Mice were immunized i.u. with Ct, UV-Ct, or UV-_Ct_–cSAP. At indicated timepoints thereafter, CD45+MHC-II+ APC subsets were isolated from uteri and LNs and FACS sorted based on CD103 and F4/80 expression. Uptake of Ct per 1,000 sorted APCs in the uterus (B) and draining LN (C) was measured by qPCR. Isolated uterine CD326+ epithelial cells (EC) served as positive control for uterine samples. Data are pooled from two independent experiments. Mac, macrophages; DC, dendritic cells. n=2-7; broken line, limit of detection; *P<0.05; **P<0.01; ***P<0.001. (D) In vitro proliferation of NR1 TN was determined by CFSE dilution after incubation with sorted APC subsets for 3 days (n=4 mice/group; *P<0.05). (E) In vivo proliferation of CFSE-labeled CD90.1+ NR1 cells in a draining popliteal LN 3 days after footpad injection of APC subsets (n=4 mice/group; *P<0.05). (F) FoxP3-eGFP-depleted NR1 cells were incubated in vitro with sorted APC subsets. Frequencies of FoxP3-eGFP+ Treg were determined by flow cytometry after 3 days (n=4 mice/group; *P<0.05). (G) 18 hours after immunization uterine DC subsets were analyzed by FACS for indicated markers (n=4). Error bars represent mean ± SEM. Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
Figure 6. Generation and protective function of uterus-resident and systemic memory T cell subsets induced by intranasal UV-_Ct_–cSAP vaccination
(A) Ct burden was determined by qPCR following i.u. Ct challenge 4 weeks after s.c. or i.n. immunization (n=5-8 mice/group). (B) Timecourse of circulating NR1 TEff during days 1-11 post immunization (n=4 mice/group). (C) Total number of NR1 cells recovered from indicated tissues on days 7 and 30 following i.n., s.c. or i.u. immunization. LN, mesenteric lymph node. (D) Timecourse of uterine NR1 cell accumulation during days 1-11 post immunization (n=4 mice/group). (E) Schematic protocol for timed neutralization of α4 integrins in the 3 groups of _UV-Ct_-cSAP vaccinated mice shown in panels F-G. (F) Total number of uterus-resident NR1 cells determined by flow cytometry (n=4 mice/group). (G) Uterine Ct burden 3 days after i.u. Ct challenge. Data are from 2 independent experiments; n=4-8 mice/group. Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
Figure 7. Mucosal UV-_Ct_–cSAP vaccination rapidly induces a transient wave of TEff that generate protective TRM in uterine mucosa
(A) Schematic protocol for timed parabiosis experiments in panel B. Parabiosis of CD90.2+CD45.2+ mice with CD90.2+CD45.1 partners was performed on indicated days before or after i.n. immunization of both partners with UV-_Ct_–cSAP. (B) On day −1, the CD45.1 animal received 1×105 CD90.1+ NR1 TN and uterine NR1 TRM were counted 6 weeks later (n=2-4 UV-_Ct_-cSAP immunized pairs and 2 naïve parabiotic pairs). (C) Schematic protocol for parabiosis experiments in panels D-G and J-K. CD90.2+CD45.2+ mice were immunized with UV-_Ct_–cSAP i.n. or UV-Ct i.u. 2 weeks before or after parabiosis with a CD90.2+CD45.1 partner. 1×105 CD90.1+ NR-1 TN were adoptively transferred to the CD45.2+ animal one day prior to immunization. Animals were either immunized 2 weeks after (group A; panels D, F, J) or two weeks before parabiosis surgery (group B; panels E, G, K). n = 3 parabiotic pairs immunized with UV-_Ct_-cSAP or UV-Ct and 2 naive parabiotic pairs. (D, E) Total number of NR1 cells in the uterus 4 days after Ct challenge (n = 3 parabiotic pairs immunized with UV-_Ct_-cSAP and 2 naïve parabiotic pairs. **P<0.01). (F-G) Ct burden 4 days after i.u. Ct challenge of both partners in animals that had been conditioned with i.n. UV-_Ct_-cSAP. (H) Schematic protocol for timed parabiosis experiments in panel I. One partner of each CD45.1/CD45.2 congenic pair was randomly chosen to be immunized on day 0 and underwent parabiosis surgery at indicated timepoints. (I) Both parabionts in each pair (n=3-4/group) were challenged i.u. with Ct on day 28. Non-immunized parabiotic pairs served as naive controls (n=4). Ct burden was assessed 6 days after challenge. All groups were significantly different from naïve parabionts (P<0.05; one-way ANOVA followed by Bonferroni's post-test.). (J, K) Uterine Ct burden 4 days after both i.u. Ct challenge of parabiotic partners after conditioning with i.n. UV-Ct. ***P<0.001; ns, non significant. Error bars show mean ± s.e.m. Unless stated otherwise, statistical differences were assessed using two-tailed _t_-test.
Figure 8. Humanized BLT mice are protected from Ct challenge after UV-_Ct_-cSAP immunization
(A, B) Immune response of a cohort of humanized BLT mice to uterine infection with Ct-E or Ct-L. All animals had been reconstituted with human fetal thymus, liver and hematopoietic stem cells from the same donor. (A) Number of IFN-γ producing human CD4 T cells per uterus (n = 5 mice/timepoint; *P<0.05). (B) Animals were infected on day 0 and rechallenged on day 21 (arrows). Uterine Ct burden was measured on days 4, 14, 21 or 25 by qPCR. LOD, limit of detection; Ct-E, C. trachomatis serotype E; Ct-L, C. trachomatis serotype L. n = 2 mice/group. *P<0.05. (C) Schematic protocol for experiments in panels D and E. These experiments were performed using two cohorts of BLT mice that had been reconstituted with material from different human donors. (D-E) BLT mice were left naive or immunized (day 0) and boosted (day 14) as indicated using Ct serotype E (_Ct_-E) as immunogen. All animals were challenged with live _Ct_-E or Ct serotype L (_Ct_-L) on day 28. Ct burden was determined (D) by qPCR or (E) by measurement of IFUs on day 6 after challenge (n=1-9 mice/group pooled from 2 independent experiments with material from genetically different human donors). *P<0.05; **P<0.01; ***P<0.001; ns, not significant. Error bars reflect mean ± SEM. (F) Schematic summary conclusion. Two waves of vaccine-induced Ct specific memory T cells must access the uterine mucosa to confer optimal protection against Ct challenge. Mucosal vaccination against Ct induces an early burst of circulating TEff (solid lines) that seed the uterine mucosa during the first week after immunization (wave 1) and give rise to long-lived resident memory T cells. By contrast, non-mucosal vaccines do not induce this first wave of mucosa-tropic memory cells (broken lines). Regardless of vaccination route, immunized hosts generate circulatory memory T cells, which do not traffic to resting uterine mucosa and are slow to access the re-challenged uterus in the absence of pre-existing TRM. However, in mucosal vaccine recipients uterine TRM can respond instantly to rechallenge with Ct and instigate the rapid recruitment of additional _Ct-_specific memory cells from the circulating pool (wave 2). Our results indicate that memory cells from both waves are required for optimal clearance of uterine Ct infection. Statistical differences were assessed using one-way ANOVA followed by Bonferroni's post-test.
Comment in
- IMMUNOLOGY. A Chlamydia vaccine on the horizon.
Brunham RC. Brunham RC. Science. 2015 Jun 19;348(6241):1322-3. doi: 10.1126/science.aac6528. Science. 2015. PMID: 26089500 No abstract available.
Similar articles
- A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct.
Rey-Ladino J, Koochesfahani KM, Zaharik ML, Shen C, Brunham RC. Rey-Ladino J, et al. Infect Immun. 2005 Mar;73(3):1568-77. doi: 10.1128/IAI.73.3.1568-1577.2005. Infect Immun. 2005. PMID: 15731055 Free PMC article. - Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae.
Nogueira CV, Zhang X, Giovannone N, Sennott EL, Starnbach MN. Nogueira CV, et al. J Immunol. 2015 Mar 1;194(5):2319-29. doi: 10.4049/jimmunol.1402675. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637024 Free PMC article. - Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis.
Rey-Ladino J, Ross AG, Cripps AW. Rey-Ladino J, et al. Hum Vaccin Immunother. 2014;10(9):2664-73. doi: 10.4161/hv.29683. Hum Vaccin Immunother. 2014. PMID: 25483666 Free PMC article. Review. - Subunit vaccines for the prevention of mucosal infection with Chlamydia trachomatis.
Yu H, Karunakaran KP, Jiang X, Brunham RC. Yu H, et al. Expert Rev Vaccines. 2016 Aug;15(8):977-88. doi: 10.1586/14760584.2016.1161510. Epub 2016 Mar 21. Expert Rev Vaccines. 2016. PMID: 26938202 Free PMC article. Review.
Cited by
- Irradiated whole cell Chlamydia vaccine confers significant protection in a murine genital tract challenge model.
Broder KC, Matrosova VY, Tkavc R, Gaidamakova EK, Ho LTVT, Macintyre AN, Soc A, Diallo A, Darnell SC, Bash S, Daly MJ, Jerse AE, Liechti GW. Broder KC, et al. NPJ Vaccines. 2024 Nov 11;9(1):207. doi: 10.1038/s41541-024-00968-z. NPJ Vaccines. 2024. PMID: 39528548 Free PMC article. - CT584 Is Not a Protective Vaccine Antigen against Respiratory Chlamydial Challenge in Mice.
Hoang-Phou S, Pal S, Slepenkin A, Abisoye-Ogunniyun A, Zhang Y, Gilmore SF, Shelby ML, Bourguet FA, Mohagheghi MV, Noy A, Rasley A, de la Maza LM, Coleman MA. Hoang-Phou S, et al. Vaccines (Basel). 2024 Oct 3;12(10):1134. doi: 10.3390/vaccines12101134. Vaccines (Basel). 2024. PMID: 39460301 Free PMC article. - A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS.
Ocón B, Xiang M, Bi Y, Tan S, Brulois K, Ayesha A, Kunte M, Zhou C, LaJevic M, Lazarus N, Mengoni F, Sharma T, Montgomery S, Hooper JE, Huang M, Handel T, Dawson JRD, Kufareva I, Zabel BA, Pan J, Butcher EC. Ocón B, et al. Nature. 2024 Sep 18. doi: 10.1038/s41586-024-08043-2. Online ahead of print. Nature. 2024. PMID: 39293486 - Chlamydia in pigs: intriguing bacteria associated with sub-clinical carriage and clinical disease, and with zoonotic potential.
Häcker G. Häcker G. Front Cell Dev Biol. 2024 Aug 14;12:1301892. doi: 10.3389/fcell.2024.1301892. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39206090 Free PMC article. Review. - Viral Vector-Based Chlamydia trachomatis Vaccines Encoding CTH522 Induce Distinct Immune Responses in C57BL/6J and HLA Transgenic Mice.
Andreacchio G, Longo Y, Moreno Mascaraque S, Anandasothy K, Tofan S, Özün E, Wilschrey L, Ptok J, Huynh DT, Luirink J, Drexler I. Andreacchio G, et al. Vaccines (Basel). 2024 Aug 22;12(8):944. doi: 10.3390/vaccines12080944. Vaccines (Basel). 2024. PMID: 39204067 Free PMC article.
References
- Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol. 2012;24:343–353. - PubMed
- Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. - PubMed
- Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. - PubMed
- Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI111595/AI/NIAID NIH HHS/United States
- T32 HL066987/HL/NHLBI NIH HHS/United States
- R01 EB000244/EB/NIBIB NIH HHS/United States
- U54 CA151884/CA/NCI NIH HHS/United States
- U54-CA119349/CA/NCI NIH HHS/United States
- AI078897/AI/NIAID NIH HHS/United States
- AI095261/AI/NIAID NIH HHS/United States
- R01 AI111595/AI/NIAID NIH HHS/United States
- R01 EB015419/EB/NIBIB NIH HHS/United States
- R01 AI062827/AI/NIAID NIH HHS/United States
- P30-AI060354/AI/NIAID NIH HHS/United States
- R00 CA160350/CA/NCI NIH HHS/United States
- U54-CA151884/CA/NCI NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- R37-EB000244/EB/NIBIB NIH HHS/United States
- P01 AI112521/AI/NIAID NIH HHS/United States
- AI069259/AI/NIAID NIH HHS/United States
- R01 AI072252/AI/NIAID NIH HHS/United States
- R01 AI069259/AI/NIAID NIH HHS/United States
- U54 CA119349/CA/NCI NIH HHS/United States
- R01 AI039558/AI/NIAID NIH HHS/United States
- U19 AI095261/AI/NIAID NIH HHS/United States
- R37 EB000244/EB/NIBIB NIH HHS/United States
- R01 AI39558/AI/NIAID NIH HHS/United States
- 1 R01-EB015419-01/EB/NIBIB NIH HHS/United States
- U19 AI113187/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials