ROBIS: A new tool to assess risk of bias in systematic reviews was developed - PubMed (original) (raw)
ROBIS: A new tool to assess risk of bias in systematic reviews was developed
Penny Whiting et al. J Clin Epidemiol. 2016 Jan.
Abstract
Objective: To develop ROBIS, a new tool for assessing the risk of bias in systematic reviews (rather than in primary studies).
Study design and setting: We used four-stage approach to develop ROBIS: define the scope, review the evidence base, hold a face-to-face meeting, and refine the tool through piloting.
Results: ROBIS is currently aimed at four broad categories of reviews mainly within health care settings: interventions, diagnosis, prognosis, and etiology. The target audience of ROBIS is primarily guideline developers, authors of overviews of systematic reviews ("reviews of reviews"), and review authors who might want to assess or avoid risk of bias in their reviews. The tool is completed in three phases: (1) assess relevance (optional), (2) identify concerns with the review process, and (3) judge risk of bias. Phase 2 covers four domains through which bias may be introduced into a systematic review: study eligibility criteria; identification and selection of studies; data collection and study appraisal; and synthesis and findings. Phase 3 assesses the overall risk of bias in the interpretation of review findings and whether this considered limitations identified in any of the phase 2 domains. Signaling questions are included to help judge concerns with the review process (phase 2) and the overall risk of bias in the review (phase 3); these questions flag aspects of review design related to the potential for bias and aim to help assessors judge risk of bias in the review process, results, and conclusions.
Conclusions: ROBIS is the first rigorously developed tool designed specifically to assess the risk of bias in systematic reviews.
Keywords: Evidence; Meta-analysis; Quality; Risk of bias; Systematic review; Tool.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
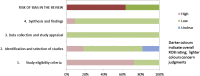
Fig. 1
Suggested graphical presentation for ROBIS results from multiple reviews.
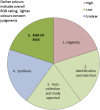
Fig. 2
Suggested graphical presentation for ROBIS results from single review: each colored segment shows the concerns for one of the phase 2 ROBIS domains; the final segment (shaded darker) shows the phase risk of bias assessment.
Comment in
- The rationale for rating risk of bias should be fully reported: response.
Whiting P, Savović J. Whiting P, et al. J Clin Epidemiol. 2016 Aug;76:239. doi: 10.1016/j.jclinepi.2016.03.008. Epub 2016 Mar 24. J Clin Epidemiol. 2016. PMID: 27034095 No abstract available. - The rationale for rating risk of bias should be fully reported.
Faggion CM Jr. Faggion CM Jr. J Clin Epidemiol. 2016 Aug;76:238. doi: 10.1016/j.jclinepi.2016.03.007. Epub 2016 Mar 24. J Clin Epidemiol. 2016. PMID: 27034096 No abstract available.
Similar articles
- [ROBIS: a new tool to assess risk of bias in systematic reviews was developed.].
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R; ROBIS Group. Whiting P, et al. Recenti Prog Med. 2018 Sep;109(9):421-431. doi: 10.1701/2990.29928. Recenti Prog Med. 2018. PMID: 30303184 Italian. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - [Risk of bias assessment: (8) Risk of Bias in Systematic Review (ROBIS)].
Hu J, Sun F, Zhan SY. Hu J, et al. Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Aug 10;39(8):1125-1129. doi: 10.3760/cma.j.issn.0254-6450.2018.08.022. Zhonghua Liu Xing Bing Xue Za Zhi. 2018. PMID: 30180441 Chinese. - A comparison of two assessment tools used in overviews of systematic reviews: ROBIS versus AMSTAR-2.
Perry R, Whitmarsh A, Leach V, Davies P. Perry R, et al. Syst Rev. 2021 Oct 25;10(1):273. doi: 10.1186/s13643-021-01819-x. Syst Rev. 2021. PMID: 34696810 Free PMC article. Review. - The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
Cited by
- Experiences of Using Cochrane Systematic Reviews by Local HTA Units.
Poder TG, Rhainds M, Bellemare CA, Deblois S, Hammana I, Safianyk C, St-Jacques S, Dagenais P. Poder TG, et al. Int J Health Policy Manag. 2022 Feb 1;11(2):112-117. doi: 10.34172/ijhpm.2020.133. Int J Health Policy Manag. 2022. PMID: 32772006 Free PMC article. Review. - Protocol for a systematic review and meta-analysis of minimal important differences for generic multiattribute utility instruments.
Henson GJ, Taylor BV, van der Mei I, Claflin SB, Simpson-Yap S, Palmer AJ, Xia Q, Antony B, Singh A, Campbell JA. Henson GJ, et al. BMJ Open. 2022 Oct 25;12(10):e062703. doi: 10.1136/bmjopen-2022-062703. BMJ Open. 2022. PMID: 36283751 Free PMC article. - Effect of Periodontal Treatment in Patients with Periodontitis and Diabetes: Review of Systematic Reviews with Meta-Analyses in the Last Five Years.
López-Valverde N, Rueda JAB. López-Valverde N, et al. Healthcare (Basel). 2024 Sep 14;12(18):1844. doi: 10.3390/healthcare12181844. Healthcare (Basel). 2024. PMID: 39337185 Free PMC article. Review. - Risk factors associated with quad bike crashes: a protocol for systematic review of observational studies.
Menon P, El-Sadig M, AB Khan M, Östlundh L, El-Deyarbi M, Al-Rifai RH, Grivna M. Menon P, et al. BMJ Open. 2021 Apr 5;11(4):e044456. doi: 10.1136/bmjopen-2020-044456. BMJ Open. 2021. PMID: 33820787 Free PMC article.
References
- Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org/ Available at. Accessed March 23, 2011.
- Centre for Reviews and Dissemination . University of York; York: 2009. Systematic Reviews: CRD's guidance for undertaking reviews in health care [Internet]http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm Available at. Accessed March 23, 2011.
- Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E., editors. Clinical practice guidelines we can trust. National Academies Press (US); Washington (DC): 2011. - PubMed
- Chandler J., Churchill R., Higgins J., Lasserson T., Tovey D. The Cochrane Collaboration; 2012. Methodological Expectations of Cochrane Intervention Reviews (MECIR): methodological standard for the conduct of new Cochrane Intervention Reviews.http://www.editorial-unit.cochrane.org/mecir Version 2.2. Available at. Accessed 17 December 2012.
- Eden J., Levit L., Berg A.O., Morton S., editors. Finding what works in health care: standards for systematic reviews. The National Academies Press; Washington, D.C: 2011. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UU_12013/9/MRC_/Medical Research Council/United Kingdom
- MR/K01465X/1/MRC_/Medical Research Council/United Kingdom
- G0902118/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources