The core spliceosome as target and effector of non-canonical ATM signalling - PubMed (original) (raw)
. 2015 Jul 2;523(7558):53-8.
doi: 10.1038/nature14512. Epub 2015 Jun 24.
Daniël O Warmerdam 2, Petros Kolovos 3, Loes Snijder 1, Mischa G Vrouwe 4, Jeroen A A Demmers 5, Wilfred F J van IJcken 6, Frank G Grosveld 3, René H Medema 2, Jan H J Hoeijmakers 1, Leon H F Mullenders 4, Wim Vermeulen 1, Jurgen A Marteijn 1
Affiliations
- PMID: 26106861
- PMCID: PMC4501432
- DOI: 10.1038/nature14512
The core spliceosome as target and effector of non-canonical ATM signalling
Maria Tresini et al. Nature. 2015.
Abstract
In response to DNA damage, tissue homoeostasis is ensured by protein networks promoting DNA repair, cell cycle arrest or apoptosis. DNA damage response signalling pathways coordinate these processes, partly by propagating gene-expression-modulating signals. DNA damage influences not only the abundance of messenger RNAs, but also their coding information through alternative splicing. Here we show that transcription-blocking DNA lesions promote chromatin displacement of late-stage spliceosomes and initiate a positive feedback loop centred on the signalling kinase ATM. We propose that initial spliceosome displacement and subsequent R-loop formation is triggered by pausing of RNA polymerase at DNA lesions. In turn, R-loops activate ATM, which signals to impede spliceosome organization further and augment ultraviolet-irradiation-triggered alternative splicing at the genome-wide level. Our findings define R-loop-dependent ATM activation by transcription-blocking lesions as an important event in the DNA damage response of non-replicating cells, and highlight a key role for spliceosome displacement in this process.
Figures
Extended data figure 1
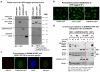
a. Schematic overview of the proteomic experiments for the identification of proteins that display UV-dependant chromatin association. b. Schematic outline of cell fractionation. c. Validation of chromatin isolation protocol for NER proteins that are reqruited to chromatin in response to DNA damage. Mock-treated or UV irradiated quiescent HDFs (20 J/m2, 1hr post-irradiation) were fractionated as outlined in (b). Equal protein amounts from each fraction were analyzed by immunobloting using antibodies against the indicated NER proteins. Abundance of H2A is shown as a control for chromatin isolation efficiency. d. UV-tiggered changes in chromatin association of core SFs, identifed by quantitative SILAC-proteomics. Proteomic experiments were performed with HDFs as outlined in (a). The table lists representative examples of SFs that participate in distinct snRNP complexes and their chromatin association in response to UV-irradiation (20 J/m2, 1h). U2 ans U5 snRNP-SFs, show signifficantly reduced chromatin association (p≤0.05, Significance B) and are indicated with a cross. ND=non-detected. e. Abundance of SFs in total cell lysates. Total lysates were prepared from U2Os cells that were mock treated or UV irradiated (20 J/m2, 1hr post-irradiation) and SFs abundance was assayed by immunobloting. Abundance of H2A is shown as a loading control. Right: immunoblots. Left: quantification of SF signal intensities normalized to H2A (n=3, mean ± S.D., one-way ANOVA / Bonferroni). f. UV-dependent interaction of splicing proteins with elongating RNAPII. Quiescent HDFs were prepared as outlined in (b) exept that, instead of MNase digestion, chromatin was mechanically sheared. Elongating RNAPII was immunoprecipitated with an antibody that recognizes specifically the ser2-phosphorylated RNAPII C-Terminal Domain (CTD) and its interaction with the U2 snRNP-SFs SF3a1 and SF3b2 was assayed by immunoblotting.
Extended data figure 2. Validation of HDFs stably expressing GFP-tagged splicing factors
a. Total cell lysates from HDFs stably expressing eGFP tagged PRP8, SF3a1, SNRNP40 or free eGFP, were analyzed by immunoblotting using antibodies against GFP (left) or against PRP8, SF3a1 and SNRNP40 (right). Ectopically produced proteins were expressed at near or below endogenous levels. b. Fluorescent microscopy images of GFP-tagged splicing factors showing the expected punctuated nuclear distribution. c. Localization of SNRNP40-GFP in nuclear speckles which were visualized by immunofluorescence detection of the speckle marker SRSF2/SC35. d. Interaction of SNRNP40-GFP with endogenous splicing factors and elongating RNAPII. Quiescent SNRNP40-GFP expressing HDFs were mock-treated or irradiated with 20 J/m2 UV-C. After a three-hour recovery period, cells were lysed under native conditions and chromatin was sheared by mechanical force. SNRNP40-GFP was immunoprecipitated from total cell lysates using GFP-TRAP® agarose beads and its association with endogenous splicing factors and the large subunit of RNAPII was assayed by immunoblotting. Untransfected cells are shown as negative control. SNRNP40-GFP interacts with U2 and U5 snRNP components, arguing that the GFP-tag does not interfere with complex formation. Interaction of SNRNP40 with its U5 snRNP partner PRP8 is partially maintained even after MNase digestion, consistent with its presence in U4/U6.U5 tri-snRNP complexes. Participation of SNRNP40-GFP in co-transcriptional splicing complexes is confirmed by co-immunoprecipitation of the active (hyperphosphorylated RNAPIIo) large subunit of RNAPII.
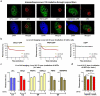
Extended data figure 3. Displacement of mature spliceosomes from subnuclear sites of UV-inflicted DNA damage
a. U2Os cells stably expressing GFP-tagged SFs were UV irradiated (60 J/m2) through isopore membranes resulting in DNA-lesion formation in small subnuclear areas. DNA damage sites (circled) were visualized by immunofluorescence using an antibody against the NER recognition factor XPC. Scale bar: 5 μm. b. SF3a1-GFP and PRP8-GFP depletion from UV-C laser micro-irradiation sites. Quantification of 20 cells from two independent experiments. eGFP localization at sites of DNA damage is used to demonstrate that depletion of eGFP-tagged SFs is not caused by photobleaching. c. UV-C laser micro-irradiation results in preferential displacement of U2 and U5-associated SFs from DNA damage sites. Quiescent HDFs were irradiated in an ≈1μm diameter nuclear area via a UV-C laser. GFP signal intensity, reflecting the abundance of GFP-tagged U1, U2, U4 and U5 snRNP components at UV-C DNA-damage sites, was quantified in the irradiated and in an unirradiated nuclear area (undamaged control). Plotted is the fluorescence signal intenisty expressed as % of that prior to irradiation, at the 1 min. time point. Cells expressing free eGFP were used as negative control. Representative from three independent experiment (n=12, mean ± s.e.m., paired T-test). d. Depletion of SFs from UV-C laser irradiation sites depends on active transcription. Transcription initiation was inhibited in quiescent HDFs by prolonged α-amanitin treatment (10 μM, ≥24h) prior to subnuclear UV-C laser irradiation. Plotted is the SNRNP40-GFP abundance in irradiated and unirradiated nuclear areas at 1 min post-irradiation. Represenative from three independent experiments (n=12, mean ± s.e.m., one-way ANOVA / Bonferroni).
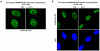
Extended data figure 4. SNRNP40 reorganization and speckle enlargement in response to UV irradiation
Representative microscopic images showing SNRNP40-GFP distribution in quiescent HDFs prior to, and one hour post UV-C irradiation with 20 J/m2. a. Live cells. b. Fixed cells.
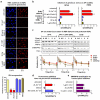
Extended data figure 5
a. RNA synthesis is inhibited preferentially by genotoxins that inflict bulky DNA lesions. Influence of genotoxins on RNA synthesis of quiescent HDFs was measured by EU-pulse labeling combined with Click chemistry. Top: representative images. Bottom: quantification of fluorescence intensity (n=150, mean ± s.e.m., one-way ANOVA / Bonferroni). b. Mobilization of U2 and U5 snRNPs by genotoxins inflicting transcription blocking-DNA lesions. Mobilization of GFP-tagged SF3a1 (left) and PRP8 (right) assayed by FRAP in quiescent HDFs exposed to different types of genotoxins (n=30, mean ± s.e.m., one-way ANOVA / Dunnett’s). c. Chromatin displacement of mature spliceosomes is not TC-NER dependent. Left: Chromatin abundance of U2 and U5 snRNP-SFs was assayed by immunoblotting in XPA deficient (left), XPA-GFP corrected (middle) and CSB deficient (right) HDFs. Cells were UV-irradiated (20 J/m2) and chromatin was isolated at the indicated times. Top: Immunoblots; bottom: quantification of SF signal intensities normalized to H2A (n=3, mean ± s.d., one-way ANOVA / Bonferroni). d. Proteasome activity is not required for UV-damage induced spliceosome mobilization. Mobilization of SNRNP40-GFP assayed by FRAP in quiescent HDFs exposed to UV radiation in the presence or absence of the proteasome inhibitor MG132 (50μM) (n=30, mean ± s.e.m., T-test). e. SNRNP40-GFP mobilization by transcription inhibition. FRAP of SNRNP40-GFP in quiescent HDFS after inhibition of transcription initiation (10 μg/ml α-amanitin, 24h) or elongation (1 μg/ml Actinomycin D or 50 μM DRB, 1h) (n=30, mean ± s.e.m., one-way ANOVA / Dunnett’s).
Extended data figure 6
a. UV-irradiation and DRB-dependent mobilization of SNRNP40. Quiescent HDFs expressing SNRNP40-GFP were UV-irradiated or DRB treated with doses that inhibit transcription to similar levels. SF mobility was assayed by FRAP. b. Additive effect of combined UV and DRB treatments. FRAP of SNRNP40-GFP in quiescent HDFs treated with DRB, UV, or a combination of both, each at a dose that inhibits RNA synthesis by ≈50%. c. Impaired UV-dependent SF3a1 mobilization in cells lacking ATM activity. SF3a1-GFP mobilization was measured by FRAP in quiescent HDFs derived from an AT patient or a healthy donor. d. ATM-dependent spliceosome mobilization. Quiescent HDFs were treated with 10 μM ATM (KU55933), ATR (VE821) or DNA-PK (NU7441) inhibitors prior to irradiation. GFP-tagged SF3a1 or PRP8 mobility was assayed by FRAP. ATM, but not ATR or DNA-PK inhibition partially prevented the UV-induced SF-mobilization. (a, b, c, d) n=25, mean ± s.e.m., one-way ANOVA / Bonferroni. e. Reduced UV-induced intron retention in response to ATM silencing. Intron inclusion in RPE cells transfected either with control or ATM silencing siRNAs and subsequently mock-treated or UV irradiated (20 J/m2, 6 hrs) was assayed by RT-PCR. f. ATM-dependent changes in intron retention. Intron inclusion was assayed by RT-PCR in untreated, UV irradiated and DRB treated quiescent cells in the presence or absence of 10 μM ATM inhibitor. g. Heatmap of UV-triggered and ATM-dependent transcriptome changes. Quiescent cells were mock-treated or UV-irradiated in the presence or absence of the ATM inhibitor. Transcriptome profiles were generated by RNA-seq. Differentially expressed genes between untreated and UV-irradiated cells (p<0.05) and UV-irradiated cells in the presence or absence of the ATM inhibitor (p<0.05), were clustered in a Heatmap using Pearson correlation. N=1676 differentially expressed transcripts. The observed anti-correlation indicates that UV-inducible transcriptome changes can be, in part, prevented by ATM inhibition. h. Lack of influence of ATM inhibition on DRB-dependent SF mobility. SF mobility was measured by FRAP in untreated or DRB treated HDFs in the presence or absence of 10 μM ATM inhibitor (n=30, mean ± s.e.m., one-way ANOVA / Bonferroni).
Extended data figure 7. Canonical and non-canonical ATM activation
a. ATM autophosphorylation (Ser 1981) was assayed in quiescent HDFs one hour after the indicated treatments. In non-replicating cells UV and Trichostatin A (TSA) activate ATM via non-canonical pathways. Transcription inhibition by DRB has no influence on ATM activity. b. The quiescent status of serum deprived HDFs was verified by immunodetection of the cell cycle marker Ki67, which is not expressed by quiescent (G0) cells. c. Immunofluorescence detection of active ATM in quiescent HDFs treated with DDR kinase inhibitors. d. Immunoblotting analysis of nuclear extracts derived from quiescent HDFs treated as in (c) using a phospho-specific ATM (S1981) antibody (top) and an antibody recognizing ATM (bottom). e. Differences in autophosphorylated-ATM distribution in quiescent HDFs treated with various ATM activators. Left: multiple cells; right: single cell magnification illustrating pan-nuclear localization of phosphorylated ATM after UV irradiation and focal accumulation after CPT or IR treatments. Magnified cells are indicated by arrows (left panel). f. Differences in amounts of DNA damage-foci formation indicative of DSBs, in response to CPT, UV-and IR. Quiescent HDFs were pre-treated with the ATR inhibitor (10 μM, 1 hr) and subsequently exposed to the indicated genotoxins. DSB-foci were visualized by immunofluorescence using antibodies against γH2A.X and p53BP1. Left: multiple cells; right: single cell magnification. Magnified cells are indicated by arrows in the left panel.
Extended data figure 8. ATM activation by interference with spliceosome assembly or RNaseH1/H2A silencing
a. ATM autophosphorylation was assayed by immunofluorescence in HDFs after silencing of SF3a1, PRP8 or combined silencing of RNaseH1 and RNaseH2A. b. Immunoblotting analysis of silenced proteins in total cell lysates. Tubulin is shown as a loading control. c. SF mobilization by the spliceosome inhibitor Pladienolide B was assayed by FRAP in quiescent HDFs. Consistent with its function in interfering with spliceosome maturation following pre-spliceosome assembly, cell treatment with pladienolide B resulted in extensive mobilization of U5 snRNP factors (PRP8 and SNRNP40), partial mobilization of the U2 snRNP SF3a1, and had no influence on the U1 snRNP factor U1A (n=30, mean ± s.e.m., one-way ANOVA / Bonferroni). d and e ATM activation by Pladienolide B. Quiescent HDFs were either treated with 5 μM Pladienolide B or exposed to 1 Gy IR and autophosphorylated ATM was detected by: (d) immunofluorescence, (e) immunoblotting. f. Effect of Pladienolide treatment on intron retention. RNA isolated from mock-treated, UV irradiated or Pladienolide B treated RPE cells. Intron retention assayed by RT-PCR on transcripts of the indicated genes, shows that Pladienolide B influences splicing to the same extend as UV-irradiation. U/S: Ratio of relative abundance of unspliced (U) to spliced (S) introns. g. Efficiency of RNaseH1 and H2A silencing at single cell level assayed by immunofluorescence.
Extended data figure 9
a. Recruitment of GFP-RNaseH1(D145N) at local DNA-damage sites depends on endogenous levels of RNaseH activity. DNA damage was inflicted via a UV-C laser in ≈1 μm-diameter subnuclear areas of cells after silencing of RNaseH2A or overexpression of RNaseH1-mCherry. Recruitment of RNaseH1(D145N)-GFP at the irradiated sites was monitored by live-cell imaging. Plotted is the fluorescence intensity of RNaseH1(D145N)-GFP at 1 min. post irradiation, at the irradiated and in a non-irradiated nuclear area. Representative from three independent experiments (n=10, mean ± s.e.m., one-way ANOVA / Bonferroni). b and c. R-loop formation at sites of local UV-C laser irradiation. Immunofluorescence detection of R-Loops using the DNA:RNA hybrid-specific S9.6 antibody. Sites of irradiation are visualized by XPC immunodetection. (b) Dashed boxes indicate the magnified areas shown in the right panels. The dashed lines indicate the line-scan track used to quantify fluorescence intensity of S9.6 and anti-XPC (shown in in the graph). (c) Specificity of the antibody was confirmed by its increased sensitivity after RNase H2A silencing and its ability to detect R-loops when suboptimal doses of UV-C irradiation were applied. d. RNaseH1 accumulation at local DNA-damage sites depends on active transcription but not ATM activity. Transcription initiation was inhibited in quiescent HDFs by α-amanitin (10 μg/ml, 24h) prior to local UV-C laser irradiation. Plotted is the fluorescence intensity at 1 min. post irradiation of RNaseH1(D145N)-GFP at the irradiated and in a non-irradiated nuclear area for untreated, ATM-inhibitor and α-amanitin treated cells. Representative from three experiments (n=10, mean ± s.e.m., one-way ANOVA / Bonferroni). e. RNaseH1 overexpression inhibits the UV-dependent spliceosome mobilization. FRAP of U2Os cells stably expressing GFP-tagged SF3a1 and PRP8 and transiently transfected with RNaseH1-mcherry. f. RNaseH1 and H2A silencing potentiates the UV-dependent spliceosome mobilization. RNaseH1 and H2 were silenced in U2Os cells expressing SF3a1-GFP or PRP8-GFP and SF mobility was assayed by FRAP. g. FRAP of SNRNP40-GFP in quiescent HDFs after RNaseH1/H2 silencing. e, f, g, n=30, mean ± s.e.m., one-way ANOVA / Bonferroni.
Extended data figure 10. Combined transcription inhibition and ATM activation, results in extensive mobilization of mature spliceosomes
a. Combinatorial effect of DRB and IR on spliceosome mobilization. Quiescent HDFs were exposed to IR in the presence or absence of DRB, and SF3a1-GFP and PRP8-GFP mobility was assayed by FRAP. b. The IR-mediated increase of DRB-dependent spliceosome mobilization depends on ATM activity. FRAP of GFP-tagged SNRNP40 in quiescent HDFs treated with DRB and/or IR in the presence or absence of an ATM inibitor. c. Spliceosome mobilization by CPT. Quiescent HDFs were treated with 25 μg/ml CPT, 25 μM DRB and 20 J/m2 UV at doses that inhibit transcription to approx. 30% and their influence on SF3a1, PRP8 and SNRNP40 mobilization was measured by FRAP. Mobilization of GFP-tagged SF3a1, PRP8 and SNRNP40 in quiescent HDFs was measured by FRAP. a, b, c, n=30, mean ± s.e.m., one-way ANOVA / Bonferroni d. Inhibition of RNA synthesis by the treatments shown in (c) was assayed in quiescent HDFs by EU-incorporation and Click-chemistry (n=150, mean ± s.e.m., one-way ANOVA / Dunnett’s).
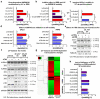
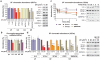
Figure 1. DNA damage-triggered chromatin-displacement of activated spliceosomes
a,b, UV-induced changes in chromatin-association of spliceosome components in quiescent HDFs; a, Immunoblots (right) and quantification (left) of SF chromatin-association; b, chromatin-associated snRNAs quantified by Q-PCR and normalized to HotAir ncRNA (n=4, mean ± s.d., T-test). d,e, immunoblots (right) and quantification (left) of SF chromatin-association in U2Os cells; d, time post UV-irradiation, e, UV dose-response and lack of influence of proteasome inhibition. b, d, e, Graphs: Signal intensities normalized to H2A. (n=3, mean ± s.d., T-test and one-way ANOVA.
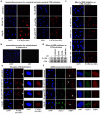
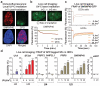
Figure 2. Mobilization and displacement of mature spliceosomes from UV-C induced DNA damage sites
a, Immunofluorescence detection of SNRNP40 and CPDs in U2Os cells UV-irradiated through porous membranes. b, SNRNP40-GFP depletion from UV-C laser microirradiation sites in U2Os cells; typical image (top) and fluoresence quantification of 20 cells (bottom). c, FRAP of UV-triggered SNRNP40-GFP mobilization in U2Os and quiescent HDFs (n=25). d, FRAP of free eGFP or GFP-tagged SFs in UV-irradiated quiescent HDFs. Δ[_mobility_] = (Fluorescence irradiated – fluorescence non-irradiated cells) at 1 min post-bleaching (n=25, mean ± s.e.m., T-test and one-way ANOVA).
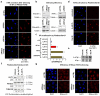
Figure 3. Chromatin displacement of mature spliceosomes is caused by RNAPII-blocking lesions and is NER-independent
a, FRAP of SNRNP40-GFP in quiescent HDFs exposed to genotoxins (n=30, mean ± s.e.m., one-way ANOVA). b, UV-triggered mobilization of SF3a1-GFP and SNRNP40-GFP in HDFs deficient in GG-NER (XP-C), TC-NER (CS-B) or both (XP-A) (n=30, mean ± s.e.m., T-test).
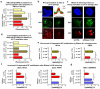
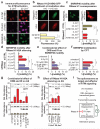
Figure 4. ATM modulates spliceosome mobilization and influences splicing decisions upon DNA damage
a, RNA synthesis measured by EU pulse-labeling. (n=150, mean ± s.e.m., T-test). b, c, d, e, FRAP of SFs in quiescent HDFs (n=25, mean ± s.e.m., one-way ANOVA); (b) response to UV- or DRB-treatment, (c) UV-irradiation +/− ATM, ATR, or DNA-PK inhibitors, (d), UV- or DRB-treatment +/− Caffeine, (e) HDFs from an AT patient or a healthy donor. f, DRB- or UV-triggered and ATM-dependent intron-inclusion assayed by RT-PCR in quiescent cells. Graphs: signal intensity expressed as unspliced/spliced ratio. (n=4, mean ± s.d., one-way ANOVA). g, Genome-wide identification by RNA-Seq, of UV-induced AS events. Left: Types of AS events. Right: number of total and ATM-dependent events.
Figure 5. Reciprocal regulation between spliceosome mobilization and R loop-dependent ATM signaling
a, Immunofluorescence of ATM activation in quiescent HDFs. b, Recruitment of RNAseH1(D145N)-GFP and mCherry-XPA at UV-C microirradiation sites (n=10, mean ± s.e.m., T-test). c,d,e,f FRAP showing SNRNP40-GFP mobilization in (c) untransfected and mCherry-RNaseH1 expressing U2OS cells, (d) after RNAseH1/H2A silencing, (e) in quiescent HDFs treated with DRB and/or IR and (f) after UV or CPT treatment. (n=30, mean ± s.e.m., one-way ANOVA). g,h, Intron retention assayed by RT-PCR in quiescent cells after (f) silencing of RNaseH1/H2A or (g) combined IR/DRB treatments (n=2, mean ± s.d., one-way ANOVA). (i) Model of UV-triggered and R-loop/ATM-augmented spliceosome mobilization.
Comment in
- DNA damage response: The spliceosome cashes in at the ATM.
Zlotorynski E. Zlotorynski E. Nat Rev Mol Cell Biol. 2015 Aug;16(8):454. doi: 10.1038/nrm4026. Epub 2015 Jul 8. Nat Rev Mol Cell Biol. 2015. PMID: 26153980 No abstract available.
Similar articles
- Bidirectional coupling of splicing and ATM signaling in response to transcription-blocking DNA damage.
Tresini M, Marteijn JA, Vermeulen W. Tresini M, et al. RNA Biol. 2016;13(3):272-8. doi: 10.1080/15476286.2016.1142039. Epub 2016 Feb 25. RNA Biol. 2016. PMID: 26913497 Free PMC article. - Noncanonical ATM Activation and Signaling in Response to Transcription-Blocking DNA Damage.
Marteijn JA, Vermeulen W, Tresini M. Marteijn JA, et al. Methods Mol Biol. 2017;1599:347-361. doi: 10.1007/978-1-4939-6955-5_25. Methods Mol Biol. 2017. PMID: 28477131 - DNA damage response: The spliceosome cashes in at the ATM.
Zlotorynski E. Zlotorynski E. Nat Rev Mol Cell Biol. 2015 Aug;16(8):454. doi: 10.1038/nrm4026. Epub 2015 Jul 8. Nat Rev Mol Cell Biol. 2015. PMID: 26153980 No abstract available. - Brd4 shields chromatin from ATM kinase signaling storms.
Choi S, Bakkenist CJ. Choi S, et al. Sci Signal. 2013 Sep 17;6(293):pe30. doi: 10.1126/scisignal.2004622. Sci Signal. 2013. PMID: 24045152 Free PMC article. Review. - DNA damage sensing by the ATM and ATR kinases.
Maréchal A, Zou L. Maréchal A, et al. Cold Spring Harb Perspect Biol. 2013 Sep 1;5(9):a012716. doi: 10.1101/cshperspect.a012716. Cold Spring Harb Perspect Biol. 2013. PMID: 24003211 Free PMC article. Review.
Cited by
- RNA: a double-edged sword in genome maintenance.
Zong D, Oberdoerffer P, Batista PJ, Nussenzweig A. Zong D, et al. Nat Rev Genet. 2020 Nov;21(11):651-670. doi: 10.1038/s41576-020-0263-7. Epub 2020 Aug 6. Nat Rev Genet. 2020. PMID: 32764716 Review. - Epigenome Maintenance in Response to DNA Damage.
Dabin J, Fortuny A, Polo SE. Dabin J, et al. Mol Cell. 2016 Jun 2;62(5):712-27. doi: 10.1016/j.molcel.2016.04.006. Mol Cell. 2016. PMID: 27259203 Free PMC article. Review. - Replication protein A binds RNA and promotes R-loop formation.
Mazina OM, Somarowthu S, Kadyrova LY, Baranovskiy AG, Tahirov TH, Kadyrov FA, Mazin AV. Mazina OM, et al. J Biol Chem. 2020 Oct 9;295(41):14203-14213. doi: 10.1074/jbc.RA120.013812. Epub 2020 Aug 12. J Biol Chem. 2020. PMID: 32796030 Free PMC article. - RNA splicing factors as oncoproteins and tumour suppressors.
Dvinge H, Kim E, Abdel-Wahab O, Bradley RK. Dvinge H, et al. Nat Rev Cancer. 2016 Jul;16(7):413-30. doi: 10.1038/nrc.2016.51. Epub 2016 Jun 10. Nat Rev Cancer. 2016. PMID: 27282250 Free PMC article. Review. - Inducible SMARCAL1 knockdown in iPSC reveals a link between replication stress and altered expression of master differentiation genes.
Pugliese GM, Salaris F, Palermo V, Marabitti V, Morina N, Rosa A, Franchitto A, Pichierri P. Pugliese GM, et al. Dis Model Mech. 2019 Oct 17;12(10):dmm039487. doi: 10.1242/dmm.039487. Dis Model Mech. 2019. PMID: 31515241 Free PMC article.
References
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. - PubMed
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Bio. 2013;14:197–210. - PubMed
METHODS REFERENCES
- Schwertman P, et al. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat Genet. 2012;44:598–+. - PubMed
- Nakazawa Y, Yamashita S, Lehmann AR, Ogi T. A semi-automated non-radioactive system for measuring recovery of RNA synthesis and unscheduled DNA synthesis using ethynyluracil derivatives. DNA Repair. 2010;9:506–516. - PubMed
- Houtsmuller AB, Vermeulen W. Macromolecular dynamics in living cell nuclei revealed by fluorescence redistribution after photobleaching. Histochem Cell Biol. 2001;115:13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 10-0594/AICR_/Worldwide Cancer Research/United Kingdom
- 233424/ERC_/European Research Council/International
- 340988/ERC_/European Research Council/International
- P01 AG017242/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous