Center Variation in Intestinal Microbiota Prior to Late-Onset Sepsis in Preterm Infants - PubMed (original) (raw)
Multicenter Study
Center Variation in Intestinal Microbiota Prior to Late-Onset Sepsis in Preterm Infants
Diana H Taft et al. PLoS One. 2015.
Abstract
Objective: Late onset sepsis (LOS) contributes to mortality and morbidity in preterm infants. We tested the hypotheses that microbes causing LOS originate from the gut, and that distortions in the gut microbial community increases subsequent risk of LOS.
Study design: We examined the gut microbial community in prospectively collected stool samples from preterm infants with LOS and an equal number of age-matched controls at two sites (Cincinnati, OH and Birmingham, AL), by sequencing the bacterial 16S rDNA. We confirmed our findings in a subset of infants by whole genome shotgun sequencing, and analyzed the data using R and LEfSe.
Results: Infants with LOS in Cincinnati, as compared to controls, had less abundant Actinobacteria in the first samples after birth (median 18 days before sepsis onset), and less abundant Pseudomonadales in the last samples collected prior to LOS (median 8 days before sepsis onset). Infants with LOS in Birmingham, as compared to controls, had no differences identified in the first sample microbial communities, but Lactobacillales was less abundant in the last samples prior to LOS (median 4 days before sepsis onset). Sequencing identified detectable levels of the sepsis-causative organism in stool samples prior to disease onset for 82% of LOS cases.
Conclusions: Translocation of gut microbes may account for the majority of LOS cases. Distortions in the fecal microbiota occur prior to LOS, but the form of distortion depends on timing and site. The microbial composition of fecal samples does not predict LOS onset in a generalizable fashion.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
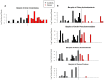
Fig 1. Barplots of taxa identified as differential abundant in cases and controls.
Y-axis was transformed by taking the base ten logarithm of the number of reads plus one. Samples were rarefied to 2000 reads per sample prior to creating the plots. A) Taxa differentially abundant by LEfSe in the first sample analysis in Birmingham. Median number of reads of Clostridiales in controls was 0, range was 0 to 5. Median number of reads of Clostridiales in cases was 2, the range was 0 to 50. Class Clostridia was also significantly different between cases and controls, it is not shown here because class Clostridia and order Clostridiales had a correlation of 1. B) Taxa differentially abundant by LEfSe in the first sample analysis in Cincinnati. Median number of reads of class Actinobacteria in controls was 1.5, range was 0 to 985. Median number of reads of class Actinobacteria in cases was 0, range was 0 to 74. Median number of reads of Pseudomonadales in controls was 2.5, range was 0 to 661. Median number of reads of Pseudomonadales in cases was 0, range was 0 to 193. Median number of reads of Acinetobacter in controls was 1.5, range was 0 to 660. Median number of reads of Acinetobacter in cases was 0, range was 0 to 75. Median number of reads of Proteus in controls was 0, range was 0 to 267. Median number of reads of Proteus in cases was 0, range was 0 to 1, Phyla Actinobacteria was also significantly different between cases and controls, it is not shown here because it has a correlation 0.9996 with class Actinobacteria and was visually indistinguishable. Family Moraxellaceae was also significantly different between cases and controls; it is not shown here because it had a correlation of 1 with genus Acinetobacter.
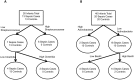
Fig 2. First sample classification tree results.
(A) Birmingham results, showing infants colonized with Streptococcaceae and infants with higher levels of Clostridia are at greater risk of sepsis. (B) Cincinnati results, showing that infants colonized with greater relative abundance of Actinobacteria and infants with lower relative abundance of Bacilli are protected from sepsis.
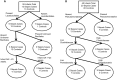
Fig 3. Last sample classification tree results for (A) Birmingham showing infants without Lactobacillales, with the unknown OTU of Bacillales, or born at younger gestational ages were more at risk of sepsis and (B) Cincinnati showing that infants with high levels of Enterobacteriales or high levels of Firmicutes were at increased risk of sepsis.
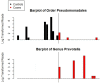
Fig 4. Barplots of taxa identified as differential abundant in cases and controls at genus level and higher in Cincinnati.
Y-axis was transformed by taking the base ten logarithm of the number of reads plus one. Samples were rarefied to 2000 reads per sample prior to creating the plots. Median number of reads of Pseudomonadales in controls was 0, range was 0 to 1251. Median number of reads of Pseudomonadales in cases was 0, range was 0 to 9. No control samples had reads of Prevotella. Median number of reads of Prevotella in cases was 0, range was 0 to 384. Family Prevotellaceae was also significantly different between cases and controls, it is not shown here because it had a correlation of 1 with genus Prevotella. All other differences identified by LEfSe were at the OTU level. All differences identified by LEfSe in the Birmingham last sample analysis were at the OTU level.
Similar articles
- The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis.
Wandro S, Osborne S, Enriquez C, Bixby C, Arrieta A, Whiteson K. Wandro S, et al. mSphere. 2018 Jun 6;3(3):e00104-18. doi: 10.1128/mSphere.00104-18. Print 2018 Jun 27. mSphere. 2018. PMID: 29875143 Free PMC article. - Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls.
Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JE, Cummings SP. Stewart CJ, et al. Microbiome. 2017 Jul 12;5(1):75. doi: 10.1186/s40168-017-0295-1. Microbiome. 2017. PMID: 28701177 Free PMC article. - Profound Pathogen-Specific Alterations in Intestinal Microbiota Composition Precede Late-Onset Sepsis in Preterm Infants: A Longitudinal, Multicenter, Case-Control Study.
El Manouni El Hassani S, Niemarkt HJ, Berkhout DJC, Peeters CFW, Hulzebos CV, van Kaam AH, Kramer BW, van Lingen RA, Jenken F, de Boode WP, Benninga MA, Budding AE, van Weissenbruch MM, de Boer NKH, de Meij TGJ. El Manouni El Hassani S, et al. Clin Infect Dis. 2021 Jul 1;73(1):e224-e232. doi: 10.1093/cid/ciaa1635. Clin Infect Dis. 2021. PMID: 33561183 - An Integrated Review of Intestinal Microbiota in the Very Premature Infant.
Dollings MC, Brown L. Dollings MC, et al. Neonatal Netw. 2016;35(4):204-16. doi: 10.1891/0730-0832.35.4.204. Neonatal Netw. 2016. PMID: 27461199 Review. - The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants.
Berkhout DJC, Niemarkt HJ, de Boer NKH, Benninga MA, de Meij TGJ. Berkhout DJC, et al. Expert Rev Gastroenterol Hepatol. 2018 May;12(5):457-470. doi: 10.1080/17474124.2018.1446826. Epub 2018 Mar 6. Expert Rev Gastroenterol Hepatol. 2018. PMID: 29488419 Review.
Cited by
- Maternal activation of the EGFR prevents translocation of gut-residing pathogenic Escherichia coli in a model of late-onset neonatal sepsis.
Knoop KA, Coughlin PE, Floyd AN, Ndao IM, Hall-Moore C, Shaikh N, Gasparrini AJ, Rusconi B, Escobedo M, Good M, Warner BB, Tarr PI, Newberry RD. Knoop KA, et al. Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7941-7949. doi: 10.1073/pnas.1912022117. Epub 2020 Mar 16. Proc Natl Acad Sci U S A. 2020. PMID: 32179676 Free PMC article. - Gut microbiota in preterm infants with late-onset sepsis and pneumonia: a pilot case-control study.
Ma Y, Peng X, Zhang J, Zhu Y, Huang R, Li G, Wu Y, Zhou C, You J, Fang S, Xiang S, Qiu J. Ma Y, et al. BMC Microbiol. 2024 Jul 22;24(1):272. doi: 10.1186/s12866-024-03419-w. BMC Microbiol. 2024. PMID: 39039501 Free PMC article. - Gut Dysbiosis, Bacterial Colonization and Translocation, and Neonatal Sepsis in Very-Low-Birth-Weight Preterm Infants.
Lee CC, Feng Y, Yeh YM, Lien R, Chen CL, Zhou YL, Chiu CH. Lee CC, et al. Front Microbiol. 2021 Oct 7;12:746111. doi: 10.3389/fmicb.2021.746111. eCollection 2021. Front Microbiol. 2021. PMID: 34690993 Free PMC article. - Adverse consequences of neonatal antibiotic exposure.
Cotten CM. Cotten CM. Curr Opin Pediatr. 2016 Apr;28(2):141-9. doi: 10.1097/MOP.0000000000000338. Curr Opin Pediatr. 2016. PMID: 26886785 Free PMC article. Review. - The Microbiota-Gut Axis in Premature Infants: Physio-Pathological Implications.
Bresesti I, Salvatore S, Valetti G, Baj A, Giaroni C, Agosti M. Bresesti I, et al. Cells. 2022 Jan 23;11(3):379. doi: 10.3390/cells11030379. Cells. 2022. PMID: 35159189 Free PMC article. Review.
References
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr., Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early human development. 2012;88 Suppl 2:S69–74. Epub 2012/05/29. 10.1016/s0378-3782(12)70019-1 - DOI - PMC - PubMed
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA: the journal of the American Medical Association. 2004;292(19):2357–65. Epub 2004/11/18. 10.1001/jama.292.19.2357 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL142708/HL/NHLBI NIH HHS/United States
- U54HG004969/HG/NHGRI NIH HHS/United States
- HHSN272200900018C/AI/NIAID NIH HHS/United States
- T32 ES 10957-11/ES/NIEHS NIH HHS/United States
- P01 HD013021/HD/NICHD NIH HHS/United States
- 5UL1RR026314-03/RR/NCRR NIH HHS/United States
- HG005969/HG/NHGRI NIH HHS/United States
- P01HD13021/HD/NICHD NIH HHS/United States
- R01 HG005969/HG/NHGRI NIH HHS/United States
- U54 HG004969/HG/NHGRI NIH HHS/United States
- R01 HD059140/HD/NICHD NIH HHS/United States
- T32 ES010957/ES/NIEHS NIH HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
- R01HD059140/HD/NICHD NIH HHS/United States
- HD27853/HD/NICHD NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- U10 HD027853/HD/NICHD NIH HHS/United States
- K08 HD084686/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical