Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial - PubMed (original) (raw)
Clinical Trial
doi: 10.1016/S1470-2045(15)00083-2. Epub 2015 Jun 23.
Igor Puzanov 2, Reinhard Dummer 3, Dirk Schadendorf 4, Omid Hamid 5, Caroline Robert 6, F Stephen Hodi 7, Jacob Schachter 8, Anna C Pavlick 9, Karl D Lewis 10, Lee D Cranmer 11, Christian U Blank 12, Steven J O'Day 13, Paolo A Ascierto 14, April K S Salama 15, Kim A Margolin 16, Carmen Loquai 17, Thomas K Eigentler 18, Tara C Gangadhar 19, Matteo S Carlino 20, Sanjiv S Agarwala 21, Stergios J Moschos 22, Jeffrey A Sosman 2, Simone M Goldinger 3, Ronnie Shapira-Frommer 8, Rene Gonzalez 10, John M Kirkwood 23, Jedd D Wolchok 24, Alexander Eggermont 6, Xiaoyun Nicole Li 25, Wei Zhou 25, Adriane M Zernhelt 25, Joy Lis 25, Scot Ebbinghaus 25, S Peter Kang 25, Adil Daud 26
Affiliations
- PMID: 26115796
- PMCID: PMC9004487
- DOI: 10.1016/S1470-2045(15)00083-2
Clinical Trial
Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial
Antoni Ribas et al. Lancet Oncol. 2015 Aug.
Abstract
Background: Patients with melanoma that progresses on ipilimumab and, if BRAF(V600) mutant-positive, a BRAF or MEK inhibitor or both, have few treatment options. We assessed the efficacy and safety of two pembrolizumab doses versus investigator-choice chemotherapy in patients with ipilimumab-refractory melanoma.
Methods: We carried out a randomised phase 2 trial of patients aged 18 years or older from 73 hospitals, clinics, and academic medical centres in 12 countries who had confirmed progressive disease within 24 weeks after two or more ipilimumab doses and, if BRAF(V600) mutant-positive, previous treatment with a BRAF or MEK inhibitor or both. Patients had to have resolution of all ipilimumab-related adverse events to grade 0-1 and prednisone 10 mg/day or less for at least 2 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and at least one measurable lesion to be eligible. Using a centralised interactive voice response system, we randomly assigned (1:1:1) patients in a block size of six to receive intravenous pembrolizumab 2 mg/kg or 10 mg/kg every 3 weeks or investigator-choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide). Randomisation was stratified by ECOG performance status, lactate dehydrogenase concentration, and BRAF(V600) mutation status. Individual treatment assignment between pembrolizumab and chemotherapy was open label, but investigators and patients were masked to assignment of the dose of pembrolizumab. We present the primary endpoint at the prespecified second interim analysis of progression-free survival in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT01704287. The study is closed to enrolment but continues to follow up and treat patients.
Findings: Between Nov 30, 2012, and Nov 13, 2013, we enrolled 540 patients: 180 patients were randomly assigned to receive pembrolizumab 2 mg/kg, 181 to receive pembrolizumab 10 mg/kg, and 179 to receive chemotherapy. Based on 410 progression-free survival events, progression-free survival was improved in patients assigned to pembrolizumab 2 mg/kg (HR 0·57, 95% CI 0·45-0·73; p<0·0001) and those assigned to pembrolizumab 10 mg/kg (0·50, 0·39-0·64; p<0·0001) compared with those assigned to chemotherapy. 6-month progression-free survival was 34% (95% CI 27-41) in the pembrolizumab 2 mg/kg group, 38% (31-45) in the 10 mg/kg group, and 16% (10-22) in the chemotherapy group. Treatment-related grade 3-4 adverse events occurred in 20 (11%) patients in the pembrolizumab 2 mg/kg group, 25 (14%) in the pembrolizumab 10 mg/kg group, and 45 (26%) in the chemotherapy group. The most common treatment-related grade 3-4 adverse event in the pembrolizumab groups was fatigue (two [1%] of 178 patients in the 2 mg/kg group and one [<1%] of 179 patients in the 10 mg/kg group, compared with eight [5%] of 171 in the chemotherapy group). Other treatment-related grade 3-4 adverse events include generalised oedema and myalgia (each in two [1%] patients) in those given pembrolizumab 2 mg/kg; hypopituitarism, colitis, diarrhoea, decreased appetite, hyponatremia, and pneumonitis (each in two [1%]) in those given pembrolizumab 10 mg/kg; and anaemia (nine [5%]), fatigue (eight [5%]), neutropenia (six [4%]), and leucopenia (six [4%]) in those assigned to chemotherapy.
Interpretation: These findings establish pembrolizumab as a new standard of care for the treatment of ipilimumab-refractory melanoma.
Funding: Merck Sharp & Dohme.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
AR reports fees to his institution from Merck for serving as an advisory board member. RD reports grant support from Bristol-Myers Squibb (BMS) and advisory board honoraria from BMS, GlaxoSmithKline (GSK), Merck Sharp & Dohme (MSD), Novartis, and Roche. DS reports grant support and study fees to his institution from Merck; personal fees for serving as an advisory board, steering committee, and speaker’s bureau member and for travel and hotel support from BMS, Merck, Novartis, and Roche-Genentech; and personal fees for advisory board and speaker’s bureau membership from Amgen and Boehringer Ingelheim. OH reports grant support from and personal fees for serving as a speaker for BMS and Merck. CR reports serving as an advisory board member for Amgen, BMS, GSK, Merck, Novartis, and Roche. FSH reports personal fees, non-financial support, and clinical trial support to his institution from Merck, grant and non-financial support and clinical trial support to his institution from BMS, and clinical trial support to his institution from Genentech. KDL reports grant support from Merck. LDC reports receiving funding to his institution from Merck for conduct of clinical trials; grant support to his institution from BMS and Genentech; and serving as a speaker programme member for BMS and Genentech. CUB reports advisory roles for BMS, GSK, MSD, Novartis, and Roche, and receipt of a research grant from Novartis. SJO’D reports receiving clinical research support from and serving as a consultant and advisory board member for Merck. PAA reports grants and personal fees from BMS, Roche-Genentech, and Ventana; personal fees and non-financial support from MSD; and personal fees from Amgen, Novartis, and GSK, all outside of the submitted work. AKSS reports grant support from BMS and personal fees for serving as a consultant for BMS and Roche-Genentech. CL reports personal fees for serving as an advisory board member from Amgen, Biontech, BMS, Celgene, MSD, and Roche; personal fees for lectures from BMS, MSD, and Roche; and personal fees for consulting from Biontech, Celgene, and Roche. TKE reports personal fees from BMS, GSK, Leo Pharma GmbH, MSD, Philogen, and Roche. TCG reports personal fees from Merck. MSC reports personal fees outside the submitted work from BMS and Merck for serving as an adviser and from BMS, GSK, Novartis, and Roche for honoraria. SJM reports support from MSD for clinical trial support and serving as an advisory board member for MSD. SMG reports personal fees and non-financial support from BMS for travel support and providing expert testimony; from MSD for travel support and advisory board membership; and research funding from the University Hospital Zurich. RG reports grant support from BMS, GSK, Merck, Novartis, and Roche, and personal fees from BMS and Roche. JMK reports personal fees from BMS, Celgene, GSK, Merck, Vical, and Ziopharm. JDW reports grant and non-financial support and personal fees from BMS and MSD. AE reports personal fees for serving on scientific advisory boards for Amgen, BMS, GSK, MedImmune, and MSD. XNL, WZ, AMZ, JL, SE, and SPK are employees of MSD. XNL, WZ, SE, and SPK hold stock in MSD. AD reports serving on advisory boards for and receiving research funding from Amgen, Genentech, GSK, OncoSec, and Roche. IP, JS, AP, KAM SSA, JAS, and RS-F declare no competing interests.
Figures
Figure 1:
Study profile
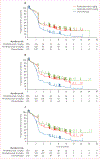
Figure 2:. Kaplan-Meier estimates of progression-free survival assessed in the intention-to-treat population
Kaplan-Meier curves for RECIST v1.1-assessed progression by independent central review (A), RECIST v1.1, by investigator review (B), and modified RECIST v1.1, by investigator review (C). RECIST v1.1=Response Evaluation Criteria in Solid Tumors, version 1.1.
Figure 3:. Prespecified subgroup analysis of progression-free survival
Progression-free survival assessed by RECIST v1.1, by independent central review in the intention-to-treat population; pembrolizumab 2 mg/kg versus chemotherapy (A) and pembrolizumab 10 mg/kg versus chemotherapy (B). HR=hazard ratio. ECOG PS=Eastern Cooperative Oncology Group performance status. LDH=lactate dehydrogenase.
Comment in
- The place of PD-1 inhibitors in melanoma management.
Bowyer S, Lorigan P. Bowyer S, et al. Lancet Oncol. 2015 Aug;16(8):873-4. doi: 10.1016/S1470-2045(15)00094-7. Epub 2015 Jun 23. Lancet Oncol. 2015. PMID: 26115798 No abstract available.
Similar articles
- Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study.
Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin JMG, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Su SC, Krepler C, Ibrahim N, Long GV. Robert C, et al. Lancet Oncol. 2019 Sep;20(9):1239-1251. doi: 10.1016/S1470-2045(19)30388-2. Epub 2019 Jul 22. Lancet Oncol. 2019. PMID: 31345627 Clinical Trial. - Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006).
Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C. Schachter J, et al. Lancet. 2017 Oct 21;390(10105):1853-1862. doi: 10.1016/S0140-6736(17)31601-X. Epub 2017 Aug 16. Lancet. 2017. PMID: 28822576 Clinical Trial. - Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma.
Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, Gonzalez R, Hodi FS, Ascierto PA, Salama AKS, Margolin KA, Gangadhar TC, Wei Z, Ebbinghaus S, Ibrahim N, Ribas A. Hamid O, et al. Eur J Cancer. 2017 Nov;86:37-45. doi: 10.1016/j.ejca.2017.07.022. Eur J Cancer. 2017. PMID: 28961465 Clinical Trial. - Pembrolizumab: A Review in Advanced Melanoma.
Deeks ED. Deeks ED. Drugs. 2016 Mar;76(3):375-86. doi: 10.1007/s40265-016-0543-x. Drugs. 2016. PMID: 26846323 Review. - The evaluation of immunotherapy and chemotherapy treatment on melanoma: a network meta-analysis.
CiRen B, Wang X, Long Z. CiRen B, et al. Oncotarget. 2016 Dec 6;7(49):81493-81511. doi: 10.18632/oncotarget.13277. Oncotarget. 2016. PMID: 27845904 Free PMC article. Review.
Cited by
- Prospective real-world study on the pharmacokinetics of pembrolizumab in patients with solid tumors.
Hurkmans DP, Sassen SDT, de Joode K, Putter L, Basak EA, Wijkhuijs AJM, Joerger M, Debets R, Koch BCP, Van der Leest CH, Schreurs MWJ, van der Veldt AAM, Aerts JGJV, Mathijssen RHJ, Koolen SLW. Hurkmans DP, et al. J Immunother Cancer. 2021 Jun;9(6):e002344. doi: 10.1136/jitc-2021-002344. J Immunother Cancer. 2021. PMID: 34088739 Free PMC article. - Using Combination therapy to overcome diverse challenges of Immune Checkpoint Inhibitors treatment.
Birnboim-Perach R, Benhar I. Birnboim-Perach R, et al. Int J Biol Sci. 2024 Jul 15;20(10):3911-3922. doi: 10.7150/ijbs.93697. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113705 Free PMC article. Review. - [Systemic treatment of inoperable metastasized malignant melanoma].
Gutzmer R, Rauschenberg R, Meier F. Gutzmer R, et al. Hautarzt. 2016 Jul;67(7):529-35. doi: 10.1007/s00105-016-3795-1. Hautarzt. 2016. PMID: 27164828 Review. German. - Clinical Predictors of Survival in Patients With BRAFV600-Mutated Metastatic Melanoma Treated With Combined BRAF and MEK Inhibitors After Immune Checkpoint Inhibitors.
Kahn AM, Perry CJ, Etts K, Kluger H, Sznol M. Kahn AM, et al. Oncologist. 2024 Apr 4;29(4):e507-e513. doi: 10.1093/oncolo/oyad300. Oncologist. 2024. PMID: 37971411 Free PMC article. - Clinical characteristics, treatment, and outcomes of pembrolizumab-induced uveitis.
Wu Z, Sun W, Wang C. Wu Z, et al. Invest New Drugs. 2024 Oct;42(5):510-517. doi: 10.1007/s10637-024-01464-w. Epub 2024 Aug 14. Invest New Drugs. 2024. PMID: 39141261
References
- McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol 2013; 31: 499–506. - PubMed
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials