Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis - PubMed (original) (raw)
Review
Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis
Vanessa J Craig et al. Am J Respir Cell Mol Biol. 2015 Nov.
Abstract
Idiopathic pulmonary fibrosis (IPF) is a restrictive lung disease that is associated with high morbidity and mortality. Current medical therapies are not fully effective at limiting mortality in patients with IPF, and new therapies are urgently needed. Matrix metalloproteinases (MMPs) are proteinases that, together, can degrade all components of the extracellular matrix and numerous nonmatrix proteins. MMPs and their inhibitors, tissue inhibitors of MMPs (TIMPs), have been implicated in the pathogenesis of IPF based upon the results of clinical studies reporting elevated levels of MMPs (including MMP-1, MMP-7, MMP-8, and MMP-9) in IPF blood and/or lung samples. Surprisingly, studies of gene-targeted mice in murine models of pulmonary fibrosis (PF) have demonstrated that most MMPs promote (rather than inhibit) the development of PF and have identified diverse mechanisms involved. These mechanisms include MMPs: (1) promoting epithelial-to-mesenchymal transition (MMP-3 and MMP-7); (2) increasing lung levels or activity of profibrotic mediators or reducing lung levels of antifibrotic mediators (MMP-3, MMP-7, and MMP-8); (3) promoting abnormal epithelial cell migration and other aberrant repair processes (MMP-3 and MMP-9); (4) inducing the switching of lung macrophage phenotypes from M1 to M2 types (MMP-10 and MMP-28); and (5) promoting fibrocyte migration (MMP-8). Two MMPs, MMP-13 and MMP-19, have antifibrotic activities in murine models of PF, and two MMPs, MMP-1 and MMP-10, have the potential to limit fibrotic responses to injury. Herein, we review what is known about the contributions of MMPs and TIMPs to the pathogenesis of IPF and discuss their potential as therapeutic targets for IPF.
Keywords: fibrosis; idiopathic pulmonary fibrosis; interstitial lung disease; lung; matrix metalloproteinase.
Figures
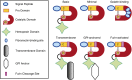
Figure 1.
Matrix metalloproteinase (MMP) classification by protein domain structure. MMPs can be categorized according to their protein domain structure. All MMPs share: (1) an N-terminal signal peptide and (2) a prodomain containing the consensus sequence in which a cysteine residue binds to the zinc ion in the catalytic domain to maintain enzyme latency. MMPs with the basic/simple domain structure also have a flexible linker region followed by a hemopexin-like domain (four-bladed β propeller structure), which helps determine substrate specificity. Minimal MMPs lack this linker and the hemopexin-like domain. Membrane-type MMPs are anchored to plasma membrane by either a transmembrane domain or a glycophosphatidyl inositol (GPI) anchor. Gelatin-binding MMPs contain fibronectin-binding sites in the catalytic domain. Furin-activated MMPs contain a furin cleavage site in their prodomain, allowing them to be activated intracellularly in the _trans_-Golgi network. C, carboxy terminus; Zn, zinc atom.
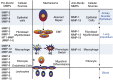
Figure 2.
Cells that express MMPs and mechanisms or potential mechanisms by which MMPs regulate pulmonary fibrosis (PF) in murine model systems. MMPs are highly expressed in lung tissues during fibrosis but vary in their dominant cellular expression (epithelial cell, fibroblast, macrophage, fibrocyte, or peripheral blood leukocyte), compartmentalization in the lung (airway and airway/alveolar epithelium, lung interstitium, or blood), and their activity (profibrotic or antifibrotic). MMPs having overall profibrotic activities (assessed as increased total lung collagen levels) in murine models of PF include MMP-3, -7, -8, -9, -12, -13, and -28 (shown in the left panel). Based upon their in vitro activities or activities in other organs, MMP-1, -2, and -11 have potential to promote PF. MMPs having overall antifibrotic activities (assessed as decreased total lung collagen levels) in models of PF include MMP-13 and -19. Based upon its in vitro activities or activities in other organs, MMP-1 and -10 have potential to inhibit PF. Antifibrotic MMPs (or potentially antifibrotic MMPs) are shown in the right panel. The mechanisms by which MMPs promote or inhibit PF are illustrated in the middle panel. EMT, epithelial mesenchymal transition; MT1, membrane type 1 metalloprotease.
Similar articles
- Matrix metalloproteinase: An upcoming therapeutic approach for idiopathic pulmonary fibrosis.
Mahalanobish S, Saha S, Dutta S, Sil PC. Mahalanobish S, et al. Pharmacol Res. 2020 Feb;152:104591. doi: 10.1016/j.phrs.2019.104591. Epub 2019 Dec 16. Pharmacol Res. 2020. PMID: 31837390 Review. - Circulating matrix metalloproteinases and tissue metalloproteinase inhibitors in patients with idiopathic pulmonary fibrosis in the multicenter IPF-PRO Registry cohort.
Todd JL, Vinisko R, Liu Y, Neely ML, Overton R, Flaherty KR, Noth I, Newby LK, Lasky JA, Olman MA, Hesslinger C, Leonard TB, Palmer SM, Belperio JA; IPF-PRO Registry investigators. Todd JL, et al. BMC Pulm Med. 2020 Mar 14;20(1):64. doi: 10.1186/s12890-020-1103-4. BMC Pulm Med. 2020. PMID: 32171287 Free PMC article. - Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis.
Nkyimbeng T, Ruppert C, Shiomi T, Dahal B, Lang G, Seeger W, Okada Y, D'Armiento J, Günther A. Nkyimbeng T, et al. PLoS One. 2013 Sep 2;8(9):e73279. doi: 10.1371/journal.pone.0073279. eCollection 2013. PLoS One. 2013. PMID: 24023851 Free PMC article. - Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis.
Pardo A, Cabrera S, Maldonado M, Selman M. Pardo A, et al. Respir Res. 2016 Mar 4;17:23. doi: 10.1186/s12931-016-0343-6. Respir Res. 2016. PMID: 26944412 Free PMC article. Review. - MMP expression and abnormal lung permeability are important determinants of outcome in IPF.
McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR. McKeown S, et al. Eur Respir J. 2009 Jan;33(1):77-84. doi: 10.1183/09031936.00060708. Epub 2008 Oct 1. Eur Respir J. 2009. PMID: 18829682
Cited by
- Spatially resolved metabolomics visualizes heterogeneous distribution of metabolites in lung tissue and the anti-pulmonary fibrosis effect of Prismatomeris connate extract.
Jiang H, Zheng B, Hu G, Kuang L, Zhou T, Li S, Chen X, Li C, Zhang D, Zhang J, Yang Z, He J, Jin H. Jiang H, et al. J Pharm Anal. 2024 Sep;14(9):100971. doi: 10.1016/j.jpha.2024.100971. Epub 2024 Mar 27. J Pharm Anal. 2024. PMID: 39381647 Free PMC article. - It takes two peroxisome proliferator-activated receptors (PPAR-β/δ and PPAR-γ) to tango idiopathic pulmonary fibrosis.
Boateng E, Bonilla-Martinez R, Ahlemeyer B, Garikapati V, Alam MR, Trompak O, Oruqaj G, El-Merhie N, Seimetz M, Ruppert C, Günther A, Spengler B, Karnati S, Baumgart-Vogt E. Boateng E, et al. Respir Res. 2024 Sep 23;25(1):345. doi: 10.1186/s12931-024-02935-7. Respir Res. 2024. PMID: 39313791 Free PMC article. - Novel Peripherally Selective Cannabinoid Receptor 1 Neutral Antagonist Improves Metabolic Dysfunction-Associated Steatotic Liver Disease in Mice.
Laudermilk LT, Schlosburg JE, Gay EA, Decker AM, Williams A, Runton R, Vasukuttan V, Kotiya A, Amato GS, Maitra R. Laudermilk LT, et al. ACS Pharmacol Transl Sci. 2024 Aug 8;7(9):2856-2868. doi: 10.1021/acsptsci.4c00356. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296275 - Idiopathic Pulmonary Fibrosis: Review of Current Knowledge.
Muri J, Durcová B, Slivka R, Vrbenská A, Makovická M, Makovický P, Škarda J, Delongová P, Kamarád V, Vecanová J. Muri J, et al. Physiol Res. 2024 Aug 31;73(4):487-497. doi: 10.33549/physiolres.935322. Physiol Res. 2024. PMID: 39264073 Free PMC article. Review. - Metabolic profiling of idiopathic pulmonary fibrosis in a mouse model: implications for pathogenesis and biomarker discovery.
Zhang YZ, Jia XJ, Xu WJ, Ding XQ, Wang XM, Chi XS, Hu Y, Yang XH. Zhang YZ, et al. Front Med (Lausanne). 2024 Aug 7;11:1410051. doi: 10.3389/fmed.2024.1410051. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39175820 Free PMC article.
References
- Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. - PubMed
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. - PubMed
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. - PubMed
- Puente XS, Sánchez LM, Overall CM, López-Otín C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL086814/HL/NHLBI NIH HHS/United States
- T32 HL007633/HL/NHLBI NIH HHS/United States
- HL105339/HL/NHLBI NIH HHS/United States
- R01 AI111475/AI/NIAID NIH HHS/United States
- R21 HL111835/HL/NHLBI NIH HHS/United States
- P01 HL114501/HL/NHLBI NIH HHS/United States
- HL111835/HL/NHLBI NIH HHS/United States
- HL063137/HL/NHLBI NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- CIA123046/PHS HHS/United States
- P01 HL105339/HL/NHLBI NIH HHS/United States
- R01 HL063137/HL/NHLBI NIH HHS/United States
- HL114501/HL/NHLBI NIH HHS/United States
- AI111475-01/AI/NIAID NIH HHS/United States
- R01 HL086814/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous