A Major Role of DNA Polymerase δ in Replication of Both the Leading and Lagging DNA Strands - PubMed (original) (raw)
A Major Role of DNA Polymerase δ in Replication of Both the Leading and Lagging DNA Strands
Robert E Johnson et al. Mol Cell. 2015.
Abstract
Genetic studies with S. cerevisiae Polδ (pol3-L612M) and Polε (pol2-M644G) mutant alleles, each of which display a higher rate for the generation of a specific mismatch, have led to the conclusion that Polε is the primary leading strand replicase and that Polδ is restricted to replicating the lagging strand template. Contrary to this widely accepted view, here we show that Polδ plays a major role in the replication of both DNA strands, and that the paucity of pol3-L612M-generated errors on the leading strand results from their more proficient removal. Thus, the apparent lack of Polδ contribution to leading strand replication is due to differential mismatch removal rather than differential mismatch generation. Altogether, our genetic studies with Pol3 and Pol2 mutator alleles support the conclusion that Polδ, and not Polε, is the major DNA polymerase for carrying out both leading and lagging DNA synthesis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
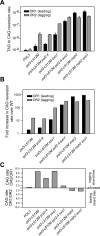
Figure 1. Orientation bias of ura3-104 reversion in the S288C pol3-L612M strains defective in Polε proofreading (pol2-4), MMR (msh2Δ) or Exo1 (exo1Δ
(A) CAG-specific reversion rates of ura3-104 for various strains in orientations OR1 and OR2. (B) Fold increase in CAG-specific reversion rates in either OR1 or OR2. (C) Reversal of strand bias in Pol3-L612M mutation generation from lagging to leading strand by inactivation of mismatch removal processes. See also Figures S1-S5.
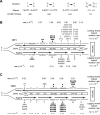
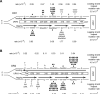
Figure 2. Lack of strand bias of URA3 mutations near ARS306 in the S288C pol3-L612M msh2Δ strain
(A) Mispair generation bias of L612M Polδ. Point mutations are shown above the two mispairs that generate the mutation. The bias of L612M Polδ for each mispair is given below (Nick McElhinney, 2007) (B) Hot spot mutations in URA3 observed in OR1 in the S288C pol3-L612M msh2Δ strain. The orientation of the URA3 ORF (boxed arrow) is depicted by the direction of the arrow. URA3, integrated ~1.2 kb to the left of ARS306 in chromosome 3 is shown schematically and is not drawn to scale. Each hot spot is shown by their respective base pairs, and their positions in the URA3 ORF are shown within the boxed arrow. Base changes generated during the replication of the leading strand (above) and the lagging strand (below) are shown. Filled in triangles represent −1 frameshift mutations. The proportion of observed mutations at each site were assigned to the lagging and leading strand according to the bias for mispair formation by L612M Polδ shown in (A). Strand specific mutation rates for each site were calculated as described in the text, and the leading and lagging strand hot spot mutation rates given on the far right are the sum of all hot spot mutations on that strand. (C) Hot spot mutations in URA3 observed in OR2 in the pol3-L612M msh2Δ strain. The orientation of URA3 is reversed from that in (B). See also Figures S2, S4, S5, and Tables S1 and S2.
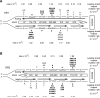
Figure 3. Lack of strand bias of URA3 mutations located near ARS306 in the DBY747 pol3-L612M msh2Δ strain
(A) Hot spot mutations in URA3 observed in OR1 in the DBY747 pol3-L612M msh2Δ strain. The URA3 coding region is integrated ~ 1.2 kb to the left of ARS306 on chromosome 3 as described in Figure 2. (B) Hot spot mutations in URA3 near ARS306 observed in OR2 in the pol3-L612M msh2Δ strain. The orientation of URA3 is reversed from that in (A). The sum of all hotspot mutations for the leading or the lagging DNA strand is given on the right. See also Figure S4 and Table S3.
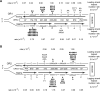
Figure 4. Lack of strand bias of URA3 mutations located ~10kb left of ARS306 in the DBY747 pol3-L612M msh2Δ strain
(A) Hot spot mutations in URA3 observed in OR1 in the DBY747 pol3-L612M msh2Δ strain. The URA3 ORF (boxed arrow) is integrated ~10 kb to the left of ARS306 on chromosome 3 in the intergenic region between the STE50 and RRP1 genes. (B) Hot spot mutations observed in URA3 located ~10 kb to the left of ARS306 in OR2 in the DBY747 pol3-L612M msh2Δ strain. The orientation of URA3 is reversed from that in (A). The sum of all hotspot mutations for the leading or the lagging DNA strand is given on the right. See also Table S4.
Figure 5. Lack of strand bias of URA3 mutations in the DBY747 pol3-L612M msh2Δ strain at ARS1
(A) Hot spot mutations in URA3 observed in OR1 in the DBY747 pol3-L612M msh2Δ strain. The URA3 ORF (boxed arrow) is located ~600 bp to the left of ARS1 on chromosome 4 and is not drawn to scale. Strand specific mutation rates for each site were calculated as described in the text, and the leading and lagging strand hot spot mutation rates given on the far right are the sum of all hot spot mutations on that strand. (B) Hot spot mutations in URA3 near ARS1 observed in OR2 in the DBY747 pol3-L612M msh2Δ strain. The orientation of URA3 is reversed from that in (A). See also Figure S7 and Tables S5 and S6.
Figure 6. Lack of pol2-M644G signature errors in MMR deficient strains
(A) Mutational bias for A to T and T to A mutations in pol2-M644G and pol2-M644G msh2Δ strains. The T:A to A:T mutation is shown above the 2 mispairs that cause the mutation. The signature bias exhibited by pol2-M644G is indicated (Pursell et al., 2007). The number of spontaneous A to T and T to A mutation events as well as hotspot mutations A279T and A686T in pol2-M644G and pol2-M644G msh2Δ strains harboring URA3 in orientation 1 (OR1) or in orientation 2 (OR2) are shown. The location of the T:dTTP mispair in the leading (lead) or lagging (lag) strand in each orientation is shown and the ratio of leading strand versus lagging strand mutations is given. (B) and (C) Schematic representation of the leading and lagging strand specific mutational events at A279 and A686 in URA3 in OR1 or in OR2 in the pol2-M644G (B) and pol2-M644G msh2Δ (C) strain.
Comment in
- Reconsidering DNA Polymerases at the Replication Fork in Eukaryotes.
Stillman B. Stillman B. Mol Cell. 2015 Jul 16;59(2):139-41. doi: 10.1016/j.molcel.2015.07.004. Mol Cell. 2015. PMID: 26186286 Free PMC article. - Who Is Leading the Replication Fork, Pol ε or Pol δ?
Burgers PMJ, Gordenin D, Kunkel TA. Burgers PMJ, et al. Mol Cell. 2016 Feb 18;61(4):492-493. doi: 10.1016/j.molcel.2016.01.017. Mol Cell. 2016. PMID: 26895421 Free PMC article. No abstract available. - Response to Burgers et al.
Johnson RE, Klassen R, Prakash L, Prakash S. Johnson RE, et al. Mol Cell. 2016 Feb 18;61(4):494-495. doi: 10.1016/j.molcel.2016.01.018. Mol Cell. 2016. PMID: 26895422 No abstract available.
Similar articles
- Exonuclease 1 preferentially repairs mismatches generated by DNA polymerase α.
Liberti SE, Larrea AA, Kunkel TA. Liberti SE, et al. DNA Repair (Amst). 2013 Feb 1;12(2):92-6. doi: 10.1016/j.dnarep.2012.11.001. Epub 2012 Dec 11. DNA Repair (Amst). 2013. PMID: 23245696 Free PMC article. - Mismatch repair operates at the replication fork in direct competition with mismatch extension by DNA polymerase δ.
Klassen R, Gangavarapu V, Johnson RE, Prakash L, Prakash S. Klassen R, et al. J Biol Chem. 2023 Apr;299(4):104598. doi: 10.1016/j.jbc.2023.104598. Epub 2023 Mar 8. J Biol Chem. 2023. PMID: 36898578 Free PMC article. - DNA polymerase δ proofreads errors made by DNA polymerase ε.
Bulock CR, Xing X, Shcherbakova PV. Bulock CR, et al. Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):6035-6041. doi: 10.1073/pnas.1917624117. Epub 2020 Mar 2. Proc Natl Acad Sci U S A. 2020. PMID: 32123096 Free PMC article. - Balancing eukaryotic replication asymmetry with replication fidelity.
Kunkel TA. Kunkel TA. Curr Opin Chem Biol. 2011 Oct;15(5):620-6. doi: 10.1016/j.cbpa.2011.07.025. Epub 2011 Aug 19. Curr Opin Chem Biol. 2011. PMID: 21862387 Free PMC article. Review. - POLD1: Central mediator of DNA replication and repair, and implication in cancer and other pathologies.
Nicolas E, Golemis EA, Arora S. Nicolas E, et al. Gene. 2016 Sep 15;590(1):128-41. doi: 10.1016/j.gene.2016.06.031. Epub 2016 Jun 16. Gene. 2016. PMID: 27320729 Free PMC article. Review.
Cited by
- The eukaryotic CMG helicase pumpjack and integration into the replisome.
Sun J, Yuan Z, Georgescu R, Li H, O'Donnell M. Sun J, et al. Nucleus. 2016 Apr 25;7(2):146-54. doi: 10.1080/19491034.2016.1174800. Nucleus. 2016. PMID: 27310307 Free PMC article. Review. - The Second Subunit of DNA Polymerase Delta Is Required for Genomic Stability and Epigenetic Regulation.
Zhang J, Xie S, Cheng J, Lai J, Zhu JK, Gong Z. Zhang J, et al. Plant Physiol. 2016 Jun;171(2):1192-208. doi: 10.1104/pp.15.01976. Epub 2016 Apr 25. Plant Physiol. 2016. PMID: 27208288 Free PMC article. - The Mechanism and Prognostic Value of DNA Polymerase δ Subunits in Hepatocellular Carcinoma: Implications for Precision Therapy.
Wang Q, Zhang S, Xu Q, Liang J, Zhang P, Huang W, Lin Z, Zheng S, Gu S, Yan J. Wang Q, et al. Int J Gen Med. 2022 Feb 9;15:1365-1380. doi: 10.2147/IJGM.S347162. eCollection 2022. Int J Gen Med. 2022. PMID: 35173474 Free PMC article. - Telomeres: Implications for Cancer Development.
Bernal A, Tusell L. Bernal A, et al. Int J Mol Sci. 2018 Jan 19;19(1):294. doi: 10.3390/ijms19010294. Int J Mol Sci. 2018. PMID: 29351238 Free PMC article. Review. - Coordinated Cut and Bypass: Replication of Interstrand Crosslink-Containing DNA.
Li Q, Dudás K, Tick G, Haracska L. Li Q, et al. Front Cell Dev Biol. 2021 Jun 28;9:699966. doi: 10.3389/fcell.2021.699966. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34262911 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases