CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function - PubMed (original) (raw)
CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function
Eunyoung Chun et al. Cell Rep. 2015.
Abstract
Our study reveals a non-canonical role for CCL2 in modulating non-macrophage, myeloid-derived suppressor cells (MDSCs) and shaping a tumor-permissive microenvironment during colon cancer development. We found that intratumoral CCL2 levels increased in patients with colitis-associated colorectal cancer (CRC), adenocarcinomas, and adenomas. Deletion of CCL2 blocked progression from dysplasia to adenocarcinoma and reduced the number of colonic MDSCs in a spontaneous mouse model of colitis-associated CRC. In a transplantable mouse model of adenocarcinoma and an APC-driven adenoma model, CCL2 fostered MDSC accumulation in evolving colonic tumors and enhanced polymorphonuclear (PMN)-MDSC immunosuppressive features. Mechanistically, CCL2 regulated T cell suppression of PMN-MDSCs in a STAT3-mediated manner. Furthermore, CCL2 neutralization decreased tumor numbers and MDSC accumulation and function. Collectively, our experiments support that perturbing CCL2 and targeting MDSCs may afford therapeutic opportunities for colon cancer interception and prevention.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
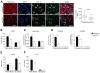
Figure 1. CCL2 Levels Increase with Neoplastic Progression in Human and Mouse Colitis-Associated CRC
(A) CCL2 expression levels in 11 human colitis-associated colorectal dysplasia and adenocarcinoma (ACA) cases. Left panel: total CCL2+ area from five fields (objective 40×) plotted for each case. Areas for quantification were no colitis (normal), colitis (no dysplasia/ACA), dysplasia, and ACA. Symbols are color-coded to track samples. Right panel: representative images of CCL2 immunostaining in human colon samples. Yellow triangles, epithelial cells; red triangles, infiltrating immune cells. Scale bar, 50 μm. (B) CCL2 colonic protein levels from _T-bet_−/− _Rag2_−/− mice across the neoplastic continuum, quantitated by ELISA. Mean ± SEM with age and number of mice per group shown. (C) Prevalence of dysplasia and neoplasia in _Ccl2_−/− _T-bet_−/− _Rag2_−/− and _T-bet_−/− _Rag2_−/− mice monitored by mouse age. Symbols represent data from individual mice, with color-coding indicating age in months. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired, two-tailed Student's t test). See also Figure S1.
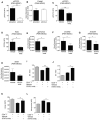
Figure 2. CCL2 Induces Colonic MDSC Accumulation and Alters MDSC Gene Expression in Colitis-Associated Pre-malignancy
Colonic myeloid cells were isolated from _Ccl2_−/− _T-bet_−/− _Rag2_−/− and _T-bet_−/− _Rag2_−/− mice with or without low-grade dysplasia (LGD). (A) The gating strategy for myeloid cell subset identification. Following a leukocytes gate (CD45+), cells are further gated for CD11b+ myeloid cells only and CD11b+Gr-1+ MDSCs or CD11b+Gr-1− macrophages (population I). Ly6C and Ly6G were used to distinguish Mo-MDSCs (population II) and PMN-MDSCs (population III). The F4/80 and MMR flow plot was used to identify tumor-promoting macrophages (TAMs and M2-like TAMs). (B) Flow cytometric analysis of populations I-III as gated in (A). Data are representative of at least three independent experiments. (C) Quantification of select colonic myeloid cells by flow cytometry. Symbols represent data from individual mice, and bars show the mean. (D) Colonic MDSC sorted from _Ccl2_−/−_T-bet_−/− _Rag2_−/− and _T-bet_−/− _Rag2_−/− mice and Arginase1 and Il10 (C), S100A8, S100A9, gp91phox, and iNOS (D) expression levels were evaluated by qRT-PCR. Symbols represent data from individual mice. Data are from age-matched (4- to 5-month old) and littermate mice. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired, two-tailed Student's t test). See also Figure S2.
Figure 3. CCL2 Levels Increase in Human Sporadic CRC and CCL2 Enhances Tumor MDSC Accumulation during Colonic Adenocarcinoma Growth
(A) CCL2 levels in human colon adenocarcinoma tissue microarrays (normal, n = 29; adenocarcinoma, n = 119). Representative images are shown. Scale bar, 500 μm. (B-F) Colon26shGFP (shControl) or Colon26shCCL2-2 (shCCL2) cells were subcutaneously injected into BALB/c mice and tumor growth evaluated at day14. (B) Representative images of tumor-bearing mice (upper panel) and tumor volumes (lower panel). (C) Representative immunofluorescence microscopy images. Tumors were stained with DAPI and for CD11b and Gr-1; single-channel and pseudocolored images. Scale bar, 50 μm. (D) Flow cytometry gating strategy and (E) analysis of intratumoral Mo-MDSCs, PMN-MDSCs, and CD11b+Gr-1− myeloid cells. (F) Intratumoral MDSC, PMN-MDSC, and Mo-MDSC numbers from shControl versus shCCL2 tumor-bearing mice with cell numbers normalized by tumor weight. Symbols represent individual mice. (G and H) shCCL2 cells were subcutaneously injected into mice and recombinant CCL2 or PBS was intratumorally injected into shCCL2 tumor-bearing mice at day 5 and day 12. Tumor volumes at day 19 (G). Intratumoral MDSC, PMN-MDSC, and Mo-MDSC numbers normalized by tumor weight (H). (I) Intratumoral TAM and M2-like-TAM cell numbers. (J) shCCL2 cells were subcutaneously injected into mice, and then sorted TAMs or MDSCs from spleens of shControl tumor-bearing mice were intratumorally injected into shCCL2 tumor-bearing mice at day 5. Tumor volumes were evaluated at day 14. All data reflect at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired, two-tailed Student's t test). See also Figure S3.
Figure 4. CCL2 Promotes T Cell Suppression via PMN-MDSCs
(A and B)T cell proliferation assays. Intratumoral PMN-MDSCs and Mo-MDSCs were sorted from shControl or shCCL2 tumor-bearing mice. (A) CellTrace-labeled splenic CD4+T cells from syngeneic mice were coincubated for 3 days with sorted intratumoral PMN-MDSCs or Mo-MDSCs. Representative flow cytometric analyses of activated or nonactivated CD4+ T cell proliferation and activated CD4+ T cell in the presence of sorted MDSCs are shown. Bar graph shows mean ± SEM of three independent experiments. (B) CellTrace-labeled splenic CD8+T cells were coincubated for 3 days with sorted intratumoral PMN-MDSCs or Mo-MDSCs. (C and D) Suppressive mechanisms of PMN-MDSCs or Mo-MDSCs on TCR components. ζ chain expression (C) and nitrotyrosine levels (D) of CD4+ T cells or CD8+ T cells coincubated with sorted intratumoral PMN-MDSCs or Mo-MDSCs were measured by flow cytometry. Data, shown as MFI ± SEM, reflect three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 (unpaired, two-tailed Student's t test). See also Figure S4.
Figure 5. CCL2 Enhances Function of PMN-MDSCs
(A) Representative images from shControl and shCCL2 tumors stained with DAPI, and for ROS (Hydro-Cy3), CD11b, and Gr-1. Single-channel pseudocolored and merged images (left panel). Scale bar, 20 μm. Microscopy-based quantitation of MFI of ROS per MDSC from shControl and shCCL2 tumors is shown (right panel). (B) ROS production levels in intratumoral PMN-MDSCs and Mo-MDSCs from shControl or shCCL2 tumor-bearing mice were detected by DCFDA staining and flow cytometry. Data are shown as MFI ± SEM. (C and D) Expression levels of gp91phox (C), S100A8 and S100A9 (D) in intratumoral PMN-MDSCs and Mo-MDSCs from shControl or shCCL2 tumors measured by RT-qPCR. Mean relative expression ± SEM are shown. (E) Expression of iNOS levels (MFI) in sorted intratumoral PMN-MDSCs or Mo-MDSCs from shControl or shCCL2 tumor-bearing mice measured by flow cytometry. (F) Expression of PD-L1 in sorted tumor-derived PMN-MDSCs or Mo-MDSCs from shControl or shCCL2 tumor-bearing mice measured by flow cytometry. Data are shown as MFI ± SEM. All data reflect three independent experiments. *p < 0.05, **p < 0.01, and ****p < 0.0001 (unpaired, two-tailed Student's t test). See also Figure S5.
Figure 6. CCL2, via STAT3, Regulates PMN-MDSC T-Cell-Suppressive Activity
(A) Phospho-STAT3 (p-STAT3) levels from PMN-MDSCs from shControl or shCCL2 tumors measured by flow cytometry. Mean ± SEM. **p < 0.001. Unpaired, two-tailed Student's t test. (B) Expression of C/ebpβ measured by qRT-PCR with data normalized to actin expression (mean relative expression ± SEM). (C–H) Analysis of sorted intratumoral PMN-MDSCs from the mice with shControl tumors followed by treatment with a STAT3 inhibitor (S3I-201) (50 μM) for 18 hr. Phospho-STAT3 (p-STAT3) levels (C) and ROS levels (DCFDA staining) (D), gp91phox (E) S100A8 (F), S100A9 (G) and iNOS (H) expression levels were measured. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01 (paired, two-tailed Student's t test). (I–L) Sorted intratumoral PMN-MDSCs from shCCL2 tumor-bearing mice were treated with S31-201 and then coincubated with CD4+ T cells or CD8+ T cells. T cell proliferation assays: CD4+ T cells (I) and CD8+ T cells (J) are shown. Division index represents T cell divisions. *p < 0.05, **p < 0.01 (paired, two-tailed Student's t test). ζ chain expression of CD4+ T cells (K) and nitrotyrosine levels of CD8+ T cells (L) measured by flow cytometry. All data represent two or three independent experiments. *p < 0.05 (paired, two-tailed Student's t test). See also Figure S6.
Figure 7. CCL2 Affects Adenoma Number and MDSC Accumulation in ApcMin/+ Mice
(A) CCL2 levels in human colon adenoma tissue microarrays (normal, n = 38; adenoma, n = 40). Representative images are shown. Scale bar, 500 μm. (B–E) ApcMin/+ mice were injected with anti-CCL2 or isotype control mAb (10 μg/kg) from 6 to 10 weeks of age twice a week for 4 weeks. (B) Photograph of intestinal tumors from representative mice (left panel). Intestinal tumor counts from ApcMin/+ mice (right panel). Symbols represent data from individual mice. (C) MDSC, PMN-MDSC, and Mo-MDSC numbers from intestinal tumors from ApcMin/+ mice treated with anti-CCL2 or isotype control mAb, with cell numbers normalized by tumor weight. (D) ROS production levels (DCFDA staining) in intratumoral PMN-MDSCs from ApcMin/+ mice treated with anti-CCL2 or isotype control mAb. (E) Expression of PD-L1 in intratumoral PMN-MDSCs from ApcMin/+ mice treated with anti-CCL2 or isotype control mAb measured by flow cytometry. (F–H) ApcMin/+ mice were injected with recombinant mouse CCL2 (20 μg/kg) from 6 to 10 weeks of age twice a week for 4 weeks or with PBS as a control. (F) Intestinal tumor counts. (G) Intestinal tumor MDSC, PMN-MDSC, and Mo-MDSC numbers. (H) PD-L1 expression in intratumoral PMN-MDSCs from ApcMin/+ mice treated with recombinant CCL2 or PBS measured by flow cytometry. All data reflect at least three independent experiments. *p < 0.05 and **p < 0.01 (unpaired, two-tailed Student's t test).
Similar articles
- Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events.
Schouppe E, Mommer C, Movahedi K, Laoui D, Morias Y, Gysemans C, Luyckx A, De Baetselier P, Van Ginderachter JA. Schouppe E, et al. Eur J Immunol. 2013 Nov;43(11):2930-42. doi: 10.1002/eji.201343349. Epub 2013 Aug 25. Eur J Immunol. 2013. PMID: 23878002 - The MUTYH base excision repair gene protects against inflammation-associated colorectal carcinogenesis.
Grasso F, Di Meo S, De Luca G, Pasquini L, Rossi S, Boirivant M, Biffoni M, Bignami M, Di Carlo E. Grasso F, et al. Oncotarget. 2015 Aug 14;6(23):19671-84. doi: 10.18632/oncotarget.4284. Oncotarget. 2015. PMID: 26109431 Free PMC article. - Intratumoral injection of mRNA encoding survivin in combination with STAT3 inhibitor stattic enhances antitumor effects.
Li M, Xie Y, Zhang J, Zhou X, Gao L, He M, Liu X, Miao X, Liu Y, Cao R, Jia Y, Zeng Z, Liu L. Li M, et al. Cancer Lett. 2024 Aug 28;598:217111. doi: 10.1016/j.canlet.2024.217111. Epub 2024 Jul 6. Cancer Lett. 2024. PMID: 38972347 - Skewing the Th cell phenotype toward Th1 alters the maturation of tumor-infiltrating mononuclear phagocytes.
Nonaka K, Saio M, Suwa T, Frey AB, Umemura N, Imai H, Ouyang GF, Osada S, Balazs M, Adany R, Kawaguchi Y, Yoshida K, Takami T. Nonaka K, et al. J Leukoc Biol. 2008 Sep;84(3):679-88. doi: 10.1189/jlb.1107729. Epub 2008 Jun 19. J Leukoc Biol. 2008. PMID: 18566103 - Role played by MDSC in colitis-associated colorectal cancer and potential therapeutic strategies.
Wang K, Wang Y, Yin K. Wang K, et al. J Cancer Res Clin Oncol. 2024 May 8;150(5):243. doi: 10.1007/s00432-024-05755-w. J Cancer Res Clin Oncol. 2024. PMID: 38717677 Free PMC article. Review.
Cited by
- Contribution of immune cells to bone metastasis pathogenesis.
He N, Jiang J. He N, et al. Front Endocrinol (Lausanne). 2022 Sep 29;13:1019864. doi: 10.3389/fendo.2022.1019864. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36246916 Free PMC article. Review. - Tumor-promoting myeloid cells in the pathogenesis of human oncoviruses: potential targets for immunotherapy.
Aghamajidi A, Farhangnia P, Pashangzadeh S, Damavandi AR, Jafari R. Aghamajidi A, et al. Cancer Cell Int. 2022 Oct 27;22(1):327. doi: 10.1186/s12935-022-02727-3. Cancer Cell Int. 2022. PMID: 36303138 Free PMC article. Review. - Metabolic Plasticity of Neutrophils: Relevance to Pathogen Responses and Cancer.
Rogers T, DeBerardinis RJ. Rogers T, et al. Trends Cancer. 2021 Aug;7(8):700-713. doi: 10.1016/j.trecan.2021.04.007. Epub 2021 May 19. Trends Cancer. 2021. PMID: 34023325 Free PMC article. Review. - C5aR1 is a master regulator in Colorectal Tumorigenesis via Immune modulation.
Ding P, Li L, Li L, Lv X, Zhou D, Wang Q, Chen J, Yang C, Xu E, Dai W, Zhang X, Wang N, Wang Q, Zhang W, Zhang L, Zhou Y, Gu H, Lei Q, Zhou X, Hu W. Ding P, et al. Theranostics. 2020 Jul 9;10(19):8619-8632. doi: 10.7150/thno.45058. eCollection 2020. Theranostics. 2020. PMID: 32754267 Free PMC article. - Hypoxia-inducible factors: a central link between inflammation and cancer.
Triner D, Shah YM. Triner D, et al. J Clin Invest. 2016 Oct 3;126(10):3689-3698. doi: 10.1172/JCI84430. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525434 Free PMC article.
References
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. - PubMed
- Bailey A, McDermott DF. Immune checkpoint inhibitors as novel targets for renal cell carcinoma therapeutics. Cancer J. 2013;19:348–352. - PubMed
- Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. - PubMed
- Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo SB, Treister NS. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol Blood Bone Marrow Transplant. 2012;18:1573–1579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous