In situ microscopic observation of chitin and fungal cells with chitinous cell walls in hydrothermal conditions - PubMed (original) (raw)
In situ microscopic observation of chitin and fungal cells with chitinous cell walls in hydrothermal conditions
Shigeru Deguchi et al. Sci Rep. 2015.
Abstract
Recent findings of intact chitin in fossil records suggest surprisingly high recalcitrance of this biopolymer during hydrothermal treatments. We also know in the experience of everyday life that mushroom, cells of which have chitinous cell walls, do not fall apart however long they are simmered. We used in situ optical microscopy to examine chitin and fungal cells with chitinous cell walls during hydrothermal treatments, and obtained direct evidence that they remained undegraded at temperatures well over 200 °C. The results show very hot and compressed water is needed to make mushrooms mushy.
Figures
Figure 1. In situ optical microscopic images showing dissolution of a flake of chitin in supercritical water.
Images were taken under a constant pressure of 25 MPa. Each image is 170 μm × 170 μm. A video clip showing the dissolution process is available in Movie S1.
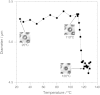
Figure 2. Change of the size of a C. liquefaciens cell as a function of temperature.
Insets show microscopic images corresponding to the temperature of the data points indicated by arrows. Each images are 26 μm × 26 μm. A video clip showing the whole process is available in Movie S2.
Figure 3. Change of the size of a C. liquefaciens cell in water between 130 °C and 310 °C as a function of temperature.
Pressure was kept constant at 25 MPa. Insets show microscopic images corresponding to the temperature of the data points indicated by arrows. Each images are 26 μm × 26 μm.
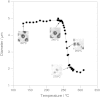
Figure 4. Morphological change of F. velutipes cells in hydrothermal conditions.
a) A series of in situ high-resolution optical microscopic images showing hyphae of F. velutipes between 100 °C and 388 °C and at a constant pressure of 25 MPa. Each images are 327 μm × 192 μm. A video clip showing the whole process is available in Movie S3. (b) Change of length (circle) and width (square) of hyphae of F. velutipes as a function of temperature.
Similar articles
- Chitinases Play a Key Role in Stipe Cell Wall Extension in the Mushroom Coprinopsis cinerea.
Zhou J, Kang L, Liu C, Niu X, Wang X, Liu H, Zhang W, Liu Z, Latgé JP, Yuan S. Zhou J, et al. Appl Environ Microbiol. 2019 Jul 18;85(15):e00532-19. doi: 10.1128/AEM.00532-19. Print 2019 Aug 1. Appl Environ Microbiol. 2019. PMID: 31126941 Free PMC article. - Fluorescence and Biochemical Assessment of the Chitin and Chitosan Content of Cryptococcus.
Maybruck BT, Upadhya R, Lam WC, Specht CA, Lodge JK. Maybruck BT, et al. Methods Mol Biol. 2024;2775:329-347. doi: 10.1007/978-1-0716-3722-7_21. Methods Mol Biol. 2024. PMID: 38758327 Free PMC article. - Solubility of (1 leads to 3)-beta-D/(1 leads to 6)-beta-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin.
Sietsma JH, Wessels JG. Sietsma JH, et al. J Gen Microbiol. 1981 Jul;125(1):209-12. doi: 10.1099/00221287-125-1-209. J Gen Microbiol. 1981. PMID: 6460846 - The structure and synthesis of the fungal cell wall.
Bowman SM, Free SJ. Bowman SM, et al. Bioessays. 2006 Aug;28(8):799-808. doi: 10.1002/bies.20441. Bioessays. 2006. PMID: 16927300 Review. - [The fungal cell wall: modern concepts of its composition and biological function ].
Feofilova EP. Feofilova EP. Mikrobiologiia. 2010 Nov-Dec;79(6):723-33. Mikrobiologiia. 2010. PMID: 21774151 Review. Russian. No abstract available.
Cited by
- Single-molecule imaging analysis reveals the mechanism of a high-catalytic-activity mutant of chitinase A from Serratia marcescens.
Visootsat A, Nakamura A, Vignon P, Watanabe H, Uchihashi T, Iino R. Visootsat A, et al. J Biol Chem. 2020 Feb 14;295(7):1915-1925. doi: 10.1074/jbc.RA119.012078. Epub 2020 Jan 10. J Biol Chem. 2020. PMID: 31924658 Free PMC article. - Nutritional, Bioactive, and Flavor Components of Giant Stropharia (Stropharia rugoso-annulata): A Review.
Huang L, He C, Si C, Shi H, Duan J. Huang L, et al. J Fungi (Basel). 2023 Jul 28;9(8):792. doi: 10.3390/jof9080792. J Fungi (Basel). 2023. PMID: 37623563 Free PMC article. Review. - Discovery of chitin in skeletons of non-verongiid Red Sea demosponges.
Ehrlich H, Shaala LA, Youssef DTA, Żółtowska-Aksamitowska S, Tsurkan M, Galli R, Meissner H, Wysokowski M, Petrenko I, Tabachnick KR, Ivanenko VN, Bechmann N, Joseph Y, Jesionowski T. Ehrlich H, et al. PLoS One. 2018 May 15;13(5):e0195803. doi: 10.1371/journal.pone.0195803. eCollection 2018. PLoS One. 2018. PMID: 29763421 Free PMC article. - Combined Approach to Engineer a Highly Active Mutant of Processive Chitinase Hydrolyzing Crystalline Chitin.
Visootsat A, Nakamura A, Wang TW, Iino R. Visootsat A, et al. ACS Omega. 2020 Oct 8;5(41):26807-26816. doi: 10.1021/acsomega.0c03911. eCollection 2020 Oct 20. ACS Omega. 2020. PMID: 33111007 Free PMC article. - First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case.
Żółtowska-Aksamitowska S, Shaala LA, Youssef DTA, Elhady SS, Tsurkan MV, Petrenko I, Wysokowski M, Tabachnick K, Meissner H, Ivanenko VN, Bechmann N, Joseph Y, Jesionowski T, Ehrlich H. Żółtowska-Aksamitowska S, et al. Mar Drugs. 2018 Feb 20;16(2):68. doi: 10.3390/md16020068. Mar Drugs. 2018. PMID: 29461501 Free PMC article.
References
- Pillai C. K. S., Paul W. & Sharma C. P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 34, 641–678 (2009).
- Souza C. P., Almeida B. C., Colwell R. R. & Rivera I. N. G. The importance of chitin in the marine environment. Mar. Biotechnol. 13, 823–830 (2011). - PubMed
- Baas M., Briggs D. E. G., Van Heemst J. D. H., Kear A. J. & De Leeuw J. W. Selective preservation of chitin during the decay of shrimp. Geochim. Cosmochim. Acta 59, 945–951 (1995).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources