Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum - PubMed (original) (raw)
Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum
T Wu et al. J Dent Res. 2015 Oct.
Abstract
Candida albicans is an opportunistic fungal pathogen found as part of the normal oral flora. It can be coisolated with Fusobacterium nucleatum, an opportunistic bacterial pathogen, from oral disease sites, such as those involved in refractory periodontitis and pulp necrosis. The physical coadherence between these 2 clinically important microbes has been well documented and suggested to play a role in facilitating their oral colonization and colocalization and contributing to polymicrobial pathogenesis. Previous studies indicated that the physical interaction between C. albicans and F. nucleatum was mediated by the carbohydrate components on the surface of C. albicans and the protein components on the Fusobaterium cell surface. However, the identities of the components involved still remain elusive. This study was aimed at identifying the genetic determinants involved in coaggregation between the 2 species. By screening a C. albicans SN152 mutant library and a panel of F. nucleatum 23726 outer membrane protein mutants, we identified FLO9, which encodes a putative adhesin-like cell wall mannoprotein of C. albicans and radD, an arginine-inhibitable adhesin-encoding gene in F. nucleatum that is involved in interspecies coadherence. Consistent with these findings, we demonstrated that the strong coaggregation between wild-type F. nucleatum 23726 and C. albicans SN152 in an in vitro assay could be greatly inhibited by arginine and mannose. Our study also suggested a complex multifaceted mechanism underlying physical interaction between C. albicans and F. nucleatum and for the first time revealed the identity of major genetic components involved in mediating the coaggregation. These observations provide useful knowledge for developing new targeted treatments for disrupting interactions between these 2 clinically relevant pathogens.
Keywords: adhesin; coaggregation; interspecies interaction; mannoprotein; opportunistic pathogen; oral microbiota.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The authors declare that W.S. and C.K. are employees of C3 Jian, Inc., which has licensed technologies from UC Regents that could be indirectly related to this research project. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
Figure 1.
Microscopic observation of coaggregation between Candida albicans (Ca) and Fusobacterium nucleatum (Fn) under different treatments. For each interacting pair, the pictures of 10 random Ca cells were taken and 1 representative image shown. The scale bar is 10 µm. wt, wild type.
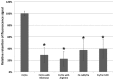
Figure 2.
Coaggregation between Candida albicans (Ca) and Fusobacterium nucleatum (Fn). Fluorescence-based coaggregation assay (as described in Materials and Methods) was performed for Fn and Ca, both wild type and mutants. The result was represented as the percentage of fluorescence signal retained compared with the control group containing wild-type Ca and Fn as a coaggregating pair. Average values ± SD are shown. Asterisk indicates the significant difference between the testing group and the control group (wild-type Fn/Ca; P < 0.05, t test). Note: The absolute number of relative fluorescence units represented by 100% was 2,500, corresponding to about 2 × 106 colony-forming units per milliliter of Fn based on the experimental procedure described in the Materials and Methods.
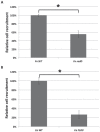
Figure 3.
Binding of Candida albicans (Ca) and Fusobacterium nucleatum (Fn) to monospecies biofilm of their interacting partner species. Recruitment assay (as described in Materials and Methods) was performed to test the ability of Fn RadD and Ca FLO9 mutant to adhere to monospecies biofilm of wild type (WT) Ca (A) and Fn (B), respectively. The result was represented as the percentage of mutant cells adhered to biofilm of its coaggregating partner compared with the WT control. Average values ± SD are shown. Asterisk indicates significant difference between the 2 sample sets (P < 0.05, t test). Note: The absolute number of cells represented by 100% was about 4 × 107 colony-forming units per milliliter for Fn (A) and 2 × 106 colony-forming units per milliliter for Ca (B) based on the experimental procedure described in Materials and Methods.
Similar articles
- Coaggregation of Candida albicans with oral Fusobacterium species.
Grimaudo NJ, Nesbitt WE. Grimaudo NJ, et al. Oral Microbiol Immunol. 1997 Jun;12(3):168-73. doi: 10.1111/j.1399-302x.1997.tb00374.x. Oral Microbiol Immunol. 1997. PMID: 9467403 - Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum.
Bor B, Cen L, Agnello M, Shi W, He X. Bor B, et al. Sci Rep. 2016 Jun 14;6:27956. doi: 10.1038/srep27956. Sci Rep. 2016. PMID: 27295972 Free PMC article. - The role of coaggregation between Porphyromonas gingivalis and Fusobacterium nucleatum on the host response to mixed infection.
Polak D, Shapira L, Weiss EI, Houri-Haddad Y. Polak D, et al. J Clin Periodontol. 2012 Jul;39(7):617-25. doi: 10.1111/j.1600-051X.2012.01889.x. Epub 2012 May 21. J Clin Periodontol. 2012. PMID: 22607053 - Fusobacterium nucleatum: an emerging gut pathogen?
Allen-Vercoe E, Strauss J, Chadee K. Allen-Vercoe E, et al. Gut Microbes. 2011 Sep 1;2(5):294-8. doi: 10.4161/gmic.2.5.18603. Epub 2011 Sep 1. Gut Microbes. 2011. PMID: 22067936 Review. - Mixed oral biofilms are controlled by the interspecies interactions of Fusobacterium nucleatum.
Valadbeigi H, Khoshnood S, Negahdari B, Maleki A, Mohammadinejat M, Haddadi MH. Valadbeigi H, et al. Oral Dis. 2024 Sep;30(6):3582-3590. doi: 10.1111/odi.14822. Epub 2023 Nov 27. Oral Dis. 2024. PMID: 38009960 Review.
Cited by
- Development of a xylose-inducible promoter and riboswitch combination system for manipulating gene expression in Fusobacterium nucleatum.
G C B, Zhou P, Naha A, Gu J, Wu C. G C B, et al. Appl Environ Microbiol. 2023 Sep 28;89(9):e0066723. doi: 10.1128/aem.00667-23. Epub 2023 Sep 11. Appl Environ Microbiol. 2023. PMID: 37695289 Free PMC article. - Chronic Inflammation as a Link between Periodontitis and Carcinogenesis.
Hoare A, Soto C, Rojas-Celis V, Bravo D. Hoare A, et al. Mediators Inflamm. 2019 Mar 27;2019:1029857. doi: 10.1155/2019/1029857. eCollection 2019. Mediators Inflamm. 2019. PMID: 31049022 Free PMC article. Review. - Identification of Fusobacterium nucleatum in primary and secondary endodontic infections and its association with clinical features by using two different methods.
Gomes BPFA, Bronzato JD, Almeida-Gomes RF, Pinheiro ET, Sousa ELR, Jacinto RC. Gomes BPFA, et al. Clin Oral Investig. 2021 Nov;25(11):6249-6258. doi: 10.1007/s00784-021-03923-7. Epub 2021 Apr 12. Clin Oral Investig. 2021. PMID: 33844080 - Insight into the Relationship between Oral Microbiota and the Inflammatory Bowel Disease.
Han Y, Wang B, Gao H, He C, Hua R, Liang C, Xin S, Wang Y, Xu J. Han Y, et al. Microorganisms. 2022 Sep 19;10(9):1868. doi: 10.3390/microorganisms10091868. Microorganisms. 2022. PMID: 36144470 Free PMC article. Review. - Microbiota and Cancer: The Emerging Beneficial Role of Bifidobacteria in Cancer Immunotherapy.
Longhi G, van Sinderen D, Ventura M, Turroni F. Longhi G, et al. Front Microbiol. 2020 Sep 8;11:575072. doi: 10.3389/fmicb.2020.575072. eCollection 2020. Front Microbiol. 2020. PMID: 33013813 Free PMC article. Review.
References
- Adam B, Baillie GS, Douglas LJ. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 51(4):344–349. - PubMed
- Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. 2012. The PATH (Prospective Antifugal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 73(4):293–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE023810/DE/NIDCR NIH HHS/United States
- R01 DE021108/DE/NIDCR NIH HHS/United States
- 1-R01-DE020102/DE/NIDCR NIH HHS/United States
- R01 DE020102/DE/NIDCR NIH HHS/United States
- 1-R01-DE023810-01/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources