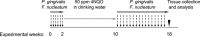Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model - PubMed (original) (raw)
Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model
Adi Binder Gallimidi et al. Oncotarget. 2015.
Abstract
Oral squamous cell carcinoma (OSCC) is a lethal disease whose incidence is increasing. Epidemiologic studies demonstrate an association between periodontitis and oral cancer, and periodontal pathogens are implicated in the pathogenesis of numerous disorders, including rheumatoid arthritis, cardiovascular diseases, diabetes and gastrointestinal malignancies. Nevertheless, a causal role for periodontal pathogens in OSCC has not been shown, partly due to the lack of an appropriate animal model. Here, utilizing a newly-established murine model of periodontitis-associated oral tumorigenesis, we report that chronic bacterial infection promotes OSCC, and that augmented signaling along the IL-6-STAT3 axis underlies this effect. Our results indicate that periodontal pathogens P. gingivalis and F. nucleatum stimulate tumorigenesis via direct interaction with oral epithelial cells through Toll-like receptors. Furthermore, oral pathogens stimulate human OSCC proliferation and induce expression of key molecules implicated in tumorigenesis. To the best of our knowledge, these findings represent the first demonstration of a mechanistic role for oral bacteria in chemically induced OSCC tumorigenesis. These results are highly relevant for the design of effective prevention and treatment strategies for OSCC.
Keywords: IL-6; STAT3; TLR2; oral cancer; periodontitis.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Figures
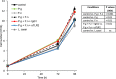
Figure 1. Schematic representation of the periodontal pathogen-associated oral tumorigenesis model
Mice were administered with oral carcinogen 4NQO (50 ppm) in the drinking water for 8 weeks (grey arrow). In some of the 4NQO-treated mice chronic periodontitis was induced by repeated oral infection with a mixture of P. gingivalis and F. nucleatum every other day, initiated 2 weeks prior to 4NQO administration, and continued (2 times/week) until week 18 (black arrows). The non-infected mice were treated with vehicle alone. At the end of experimental week 18 mice were sacrificed (black arrowhead), their tongues harvested and processed for histological examination and immunostaining.
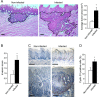
Figure 2. Increased tumor severity in P. gingivalis/F. nucleatum infected mice
A, B. Serial tongue sections (5 μm thick) harvested from 4NQO-treated, non-infected and infected mice on week 18 were stained with H&E and visualized with a Zeiss axioscope microscope. A. The area of each neoplastic lesion was determined with Axio Vision Image software. Left, middle. Representative microphotographs of H&E stained tongue sections. Tumors are delineated by dashed lines; Scale bars: 200 μm. Right. Average tumor area in non-infected (empty bar) and infected (black bar) mice on experimental week 18. Data are the mean ± SE, n = 5 mice per condition; *p = 0.004 (Mann-Whitney). B. Invasive score of the tumors found in non-infected (empty bar) and infected (black bar) mice was determined as described in Methods and in Supplementary Figure 1. Data are the mean ± SE, n = 7 mice per condition, *p = 0.029 (Mann-Whitney). C, D. Immunostaining with anti cyclin D1 antibody reveals enhanced expression of cyclin D1 in non-cancerous (top) and cancerous (bottom) tongue epithelium of infected vs. non-infected mice. C. Representative images of cyclin D1 immunostained sections are shown; Scale bars: 100 μm. D. Percentage of Cyclin D1-immunostained cells in the epithelium was quantified as the number of positively stained cells per existing total epithelial cells, based on [58] in 5 randomly selected high-power microscopic fields per mouse at magnification × 200. Data are the mean ± SE, n = 4 mice per condition *p = 0.0026 (Student's _t_-test).
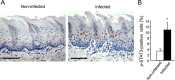
Figure 3. Increased STAT3 activation in tongue epithelium of infected mice
C57/Bl6 mice were infected with a mixture of P. gingivalis and F. nucleatum (as described in Methods) every other day for 6 days. Mice were sacrificed twenty-four hours after the last infection, their tongues removed and processed for immunostaining with anti-pSTAT3 antibody. A. Immunostaining with anti pSTAT3 antibody reveals increased levels of nuclear-localized pSTAT3 in tongue epithelium of infected (right) vs. non-infected (left) mice. Representative images of are shown. Magnification × 200 B. Percentage of pSTAT3-immunostained cells in the epithelium was calculated in ≥5 microscopic fields per mouse. Data are the mean ± SE, *p = 0.01 (Student's _t_-test). Scale bars: 100 μm.
Figure 4. Increased IL-6 levels in tongue epithelium of infected mice
A. Mice non-infected (white bar) or infected (black bar) with a mixture of P. gingivalis and F. nucleatum every other day for 6 days (as described in Methods) were sacrificed four hours after the last infection. The tongue mucosa was isolated and processed for RNA. Quantitative RT-PCR analysis revealed a 3 fold increase in IL-6 mRNA levels in the tongue mucosa of infected mice. Data are the mean ± SE, *p = 0.014 (Student's _t_-test). B. Immuno-histochemical analysis with anti-IL-6 antibody revealed expression of IL-6 protein in epithelial compartment of the tongue tissues harvested non-infected and infected mice treated with 4NQO as described in Methods and in Figure 1. Scale bars: 100 μm.
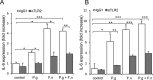
Figure 5. Increased expression of IL-6 in oral cavity SCC cell lines following co-culture with P. gingivalis and F. nucleatum
SCC-25 A. and CAL 27 B. cells were cultured alone (control) or with P. gingivalis (P.g), F. nucleatum (F.n), or mixture of P. gingivalis and F. nucleatum (P.g + F.n), as described in Methods. In some plates neutralizing antibody directed against TLR2 (αTLR2) was added to SCC-25 (A, filled bars) and CAL 27 (B, filled bars) cells, cultured alone (control) or with P.g, F.n, or mixture of P.g + F.n as described in Methods. Empty bars: isotype control (IgG1). Quantitative RT-PCR was used to assess expression of IL-6. Data are the mean ± SE; *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6. P. gingivalis and F. nucleatum stimulate in vitro proliferation of human SCC cells
SCC-25 cells were plated on 24-well plates at 104 cells per well (in quadruplicates) and cultured either alone (control) or in the presence of P. gingivalis (P.g), F. nucleatum (F.n), mixture of both (P.g + F.n), or L. Casei, as described in Methods. In some plates αTLR2 or the isotype control (IgG1) was added one hour prior to mixture of P.g + F.n. Cell numbers were determined as described in Methods. Data are the mean ± SE. Inset: p values determined by Student's _t_-test.
Similar articles
- The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma.
Chang C, Geng F, Shi X, Li Y, Zhang X, Zhao X, Pan Y. Chang C, et al. Appl Microbiol Biotechnol. 2019 Feb;103(3):1393-1404. doi: 10.1007/s00253-018-9475-6. Epub 2018 Nov 23. Appl Microbiol Biotechnol. 2019. PMID: 30470868 - About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview.
Ciani L, Libonati A, Dri M, Pomella S, Campanella V, Barillari G. Ciani L, et al. Int J Mol Sci. 2024 May 7;25(10):5083. doi: 10.3390/ijms25105083. Int J Mol Sci. 2024. PMID: 38791123 Free PMC article. Review. - Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin.
Kamarajan P, Ateia I, Shin JM, Fenno JC, Le C, Zhan L, Chang A, Darveau R, Kapila YL. Kamarajan P, et al. PLoS Pathog. 2020 Oct 1;16(10):e1008881. doi: 10.1371/journal.ppat.1008881. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33002094 Free PMC article. - Serum Levels of Interleukin-6 and Titers of Antibodies Against Porphyromonas gingivalis Could Be Potential Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma.
Park DG, Woo BH, Lee BJ, Yoon S, Cho Y, Kim YD, Park HR, Song JM. Park DG, et al. Int J Mol Sci. 2019 Jun 4;20(11):2749. doi: 10.3390/ijms20112749. Int J Mol Sci. 2019. PMID: 31167516 Free PMC article. - Role of oral microbiome in oral oncogenesis, tumor progression, and metastasis.
Li R, Xiao L, Gong T, Liu J, Li Y, Zhou X, Li Y, Zheng X. Li R, et al. Mol Oral Microbiol. 2023 Feb;38(1):9-22. doi: 10.1111/omi.12403. Epub 2022 Dec 10. Mol Oral Microbiol. 2023. PMID: 36420924 Review.
Cited by
- Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions.
Baxter NT, Ruffin MT 4th, Rogers MA, Schloss PD. Baxter NT, et al. Genome Med. 2016 Apr 6;8(1):37. doi: 10.1186/s13073-016-0290-3. Genome Med. 2016. PMID: 27056827 Free PMC article. - Subgingival Periopathogens Assessment and Clinical Periodontal Evaluation of Gastric Cancer Patients-A Cross Sectional Pilot Study.
Nicolae FM, Didilescu AC, Șurlin P, Ungureanu BS, Șurlin VM, Pătrașcu Ș, Ramboiu S, Jelihovschi I, Iancu LS, Ghilusi M, Cucu M, Gheonea DI. Nicolae FM, et al. Pathogens. 2022 Mar 16;11(3):360. doi: 10.3390/pathogens11030360. Pathogens. 2022. PMID: 35335684 Free PMC article. - The Heparanase Regulatory Network in Health and Disease.
Mayfosh AJ, Nguyen TK, Hulett MD. Mayfosh AJ, et al. Int J Mol Sci. 2021 Oct 14;22(20):11096. doi: 10.3390/ijms222011096. Int J Mol Sci. 2021. PMID: 34681753 Free PMC article. Review. - Common Risk Factor Approach to Limit Noncommunicable Diseases and Periodontal Disease-The Molecular and Cellular Basis: A Narrative Review.
Puzhankara L, Janakiram C. Puzhankara L, et al. J Int Soc Prev Community Dent. 2021 Sep 21;11(5):490-502. doi: 10.4103/jispcd.JISPCD_109_21. eCollection 2021 Sep-Oct. J Int Soc Prev Community Dent. 2021. PMID: 34760792 Free PMC article. Review. - Microbiomes, Their Function, and Cancer: How Metatranscriptomics Can Close the Knowledge Gap.
Aitmanaitė L, Širmonaitis K, Russo G. Aitmanaitė L, et al. Int J Mol Sci. 2023 Sep 7;24(18):13786. doi: 10.3390/ijms241813786. Int J Mol Sci. 2023. PMID: 37762088 Free PMC article. Review.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
- Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299. - PubMed
- Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res. 2013;19:2834–2841. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous