Regulation of mTORC1 by PI3K signaling - PubMed (original) (raw)
Review
Regulation of mTORC1 by PI3K signaling
Christian C Dibble et al. Trends Cell Biol. 2015 Sep.
Abstract
The class I phosphoinositide 3-kinase (PI3K)-mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) signaling network directs cellular metabolism and growth. Activation of mTORC1 [composed of mTOR, regulatory-associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein 8(mLST8), 40-kDa proline-rich Akt substrate (PRAS40), and DEP domain-containing mTOR-interacting protein (DEPTOR)] depends on the Ras-related GTPases (Rags) and Ras homolog enriched in brain (Rheb) GTPase and requires signals from amino acids, glucose, oxygen, energy (ATP), and growth factors (including cytokines and hormones such as insulin). Here we discuss the signal transduction mechanisms through which growth factor-responsive PI3K signaling activates mTORC1. We focus on how PI3K-dependent activation of Akt and spatial regulation of the tuberous sclerosis complex (TSC) complex (TSC complex) [composed of TSC1, TSC2, and Tre2-Bub2-Cdc16-1 domain family member 7 (TBC1D7)] switches on Rheb at the lysosome, where mTORC1 is activated. Integration of PI3K- and amino acid-dependent signals upstream of mTORC1 at the lysosome is detailed in a working model. A coherent understanding of the PI3K-mTORC1 network is imperative as its dysregulation has been implicated in diverse pathologies including cancer, diabetes, autism, and aging.
Keywords: Rag; Raptor; Rheb; TSC2; insulin; lysosome.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
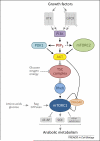
Figure 1
The primary pathway through which class I PI3K activates mTORC1. Growth factors (including hormones, cytokines, and chemokines) activate RTKs or GPCRs which, through a variety of mechanisms, activate PI3K. PI3K generates PIP3 which specifically binds Akt and PDK1 promoting the phosphorylation and activation of Akt by PDK1. Phosphorylation of Akt by mTORC2 boosts its activity several-fold and mTORC2 activation is at least partially PI3K-dependent. Akt inhibits the TSC complex, the specific GAP for the small GTPase Rheb, through multi-site phosphorylation of the TSC2 subunit. This relieves inhibition of Rheb, allowing it to become activate and stimulate mTORC1 kinase activity. Once mTORC1 is activated by Rheb, the simultaneous phosphorylation of its inhibitory subunit PRAS40 by Akt and mTORC1 itself causes PRAS40 to dissociate from mTORC1. This is thought to increase substrate access to the complex. Glucose, oxygen, and energy levels are also sensed upstream of the TSC complex. Amino acids (and glucose) are sensed upstream of mTORC1 via pathways that regulate the Rag GTPases, which do not directly activate mTORC1 but serve to bring it into proximity with Rheb in cells. mTORC1 directly phosphorylates numerous substrates including S6K and 4E-BP which mediate its control of anabolic metabolism, cellular growth, and proliferation.
Figure 2
A working model of mTORC1 activation by PI3K signaling and amino acids at the lysosome. Proper activation of mTORC1 requires both the Rag and Rheb GTPases, which are activated by amino acids and growth factors respectively. These signals are integrated at the cytoplasmic face of the lysosomal membrane where mTORC1 is activated. In the absence of amino acids, mTORC1 localizes away from the lysosome. Amino acid signaling activates the Rag GTPases which directly recruit mTORC1 to the lysosome but do not stimulate its kinase activity. The Rags, which function as heterodimers consisting of A or B with C or D isoforms, associate with the lysosome through their interaction with the membrane-tethered Ragulator complex. The Ragulator also associates with integral membrane proteins and complexes of the lysosome that participate in amino acid sensing (light green, unlabeled). A variety of other amino acid-sensitive regulators of mTORC1 have been identified but are not shown. In the absence of growth factors, the TSC complex, which is the GAP for Rheb, localizes to the lysosome in a Rheb-dependent manner and keeps Rheb inactive. Stimulation with growth factors leads to the PI3K-dependent activation of Akt with then phosphorylates TSC2 on multiple sites within the TSC complex. These phosphorylation events induce dissociation of the intact TSC complex from the lysosome and Rheb, allowing Rheb to become active and directly simulate mTORC1. Hence amino acid signaling to the Rags and growth factor-PI3K signaling to Rheb represent parallel, independent inputs that are each necessary but no sufficient for full activation of mTORC1. The TSC complex is a large oligomeric structure, composed of approximately five copies of each subunit (TSC2, TSC2, and TBC1D7) (inset box). In addition to functioning as a GAP for Rheb and converting it to its inactive GDP-bound state, the TSC complex might also prevent Rheb from activating mTORC1 by sequestering its GDP-bound form. Basal activation of either the Rags or Rheb may result in incomplete effects of amino acid or growth factor starvations on mTORC1 activation.
Similar articles
- RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells.
Gordon BS, Kazi AA, Coleman CS, Dennis MD, Chau V, Jefferson LS, Kimball SR. Gordon BS, et al. Cell Signal. 2014 Mar;26(3):461-7. doi: 10.1016/j.cellsig.2013.11.035. Epub 2013 Dec 3. Cell Signal. 2014. PMID: 24316235 Free PMC article. - Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity.
Carroll B, Maetzel D, Maddocks OD, Otten G, Ratcliff M, Smith GR, Dunlop EA, Passos JF, Davies OR, Jaenisch R, Tee AR, Sarkar S, Korolchuk VI. Carroll B, et al. Elife. 2016 Jan 7;5:e11058. doi: 10.7554/eLife.11058. Elife. 2016. PMID: 26742086 Free PMC article. - Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome.
Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Menon S, et al. Cell. 2014 Feb 13;156(4):771-85. doi: 10.1016/j.cell.2013.11.049. Cell. 2014. PMID: 24529379 Free PMC article. - Amino acid regulation of TOR complex 1.
Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Avruch J, et al. Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review. - Mammalian target of rapamycin and tuberous sclerosis complex.
Wataya-Kaneda M. Wataya-Kaneda M. J Dermatol Sci. 2015 Aug;79(2):93-100. doi: 10.1016/j.jdermsci.2015.04.005. Epub 2015 Apr 25. J Dermatol Sci. 2015. PMID: 26051878 Review.
Cited by
- Polygenic risk score model for renal cell carcinoma in the Korean population and relationship with lifestyle-associated factors.
Hong JY, Han JH, Jeong SH, Kwak C, Kim HH, Jeong CW. Hong JY, et al. BMC Genomics. 2024 Jan 10;25(1):46. doi: 10.1186/s12864-024-09974-w. BMC Genomics. 2024. PMID: 38200428 Free PMC article. - The molecular genetics of PI3K/PTEN/AKT/mTOR pathway in the malformations of cortical development.
Ma Q, Chen G, Li Y, Guo Z, Zhang X. Ma Q, et al. Genes Dis. 2023 Jul 16;11(5):101021. doi: 10.1016/j.gendis.2023.04.041. eCollection 2024 Sep. Genes Dis. 2023. PMID: 39006182 Free PMC article. Review. - Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes.
Takahara T, Amemiya Y, Sugiyama R, Maki M, Shibata H. Takahara T, et al. J Biomed Sci. 2020 Aug 17;27(1):87. doi: 10.1186/s12929-020-00679-2. J Biomed Sci. 2020. PMID: 32799865 Free PMC article. Review. - Different metabolic responses to PI3K inhibition in NSCLC cells harboring wild-type and G12C mutant KRAS.
Caiola E, Brunelli L, Marabese M, Broggini M, Lupi M, Pastorelli R. Caiola E, et al. Oncotarget. 2016 Aug 9;7(32):51462-51472. doi: 10.18632/oncotarget.9849. Oncotarget. 2016. PMID: 27283493 Free PMC article. - CHIR99021 Augmented the Function of Late Endothelial Progenitor Cells by Preventing Replicative Senescence.
Rethineswaran VK, Kim DY, Kim YJ, Jang W, Ji ST, Van LTH, Giang LTT, Ha JS, Yun J, Jung J, Kwon SM. Rethineswaran VK, et al. Int J Mol Sci. 2021 Apr 30;22(9):4796. doi: 10.3390/ijms22094796. Int J Mol Sci. 2021. PMID: 33946516 Free PMC article.
References
- Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40:88–100. - PubMed
- Vanhaesebroeck B, et al. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous